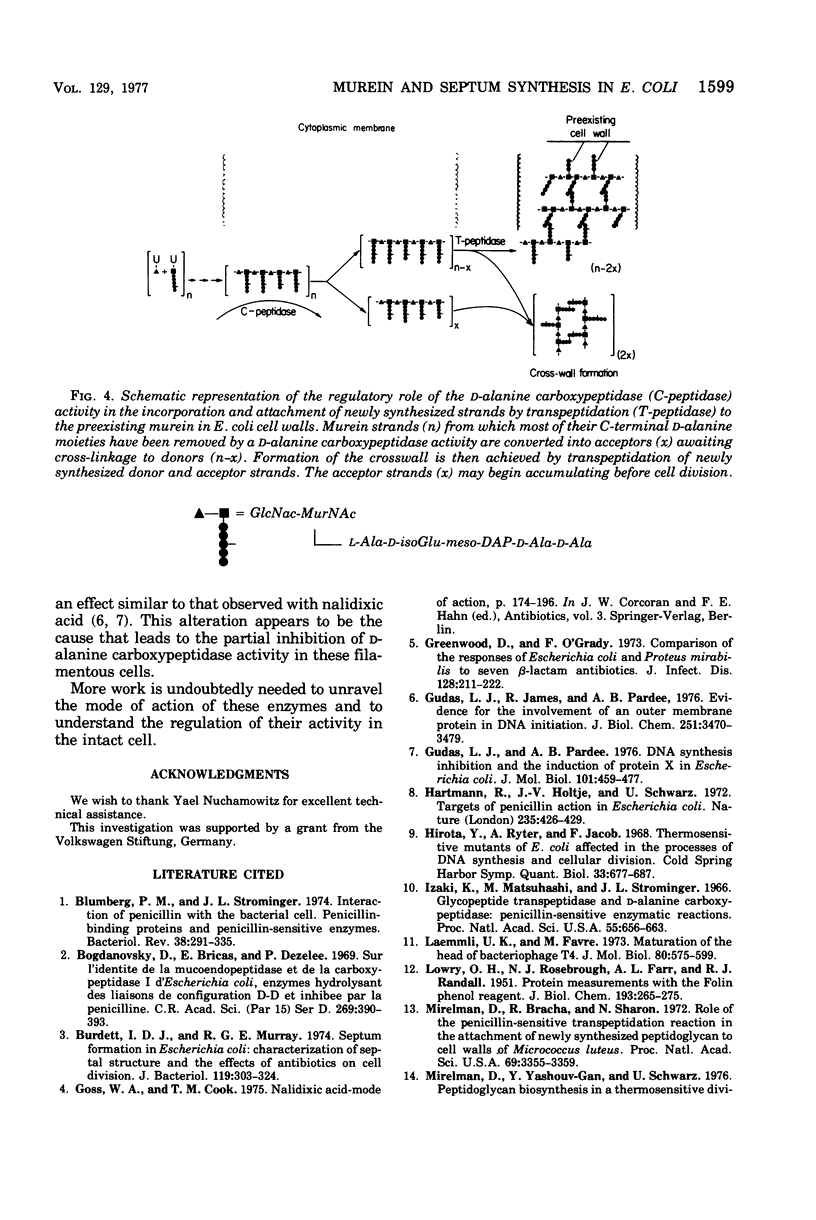
Abstract
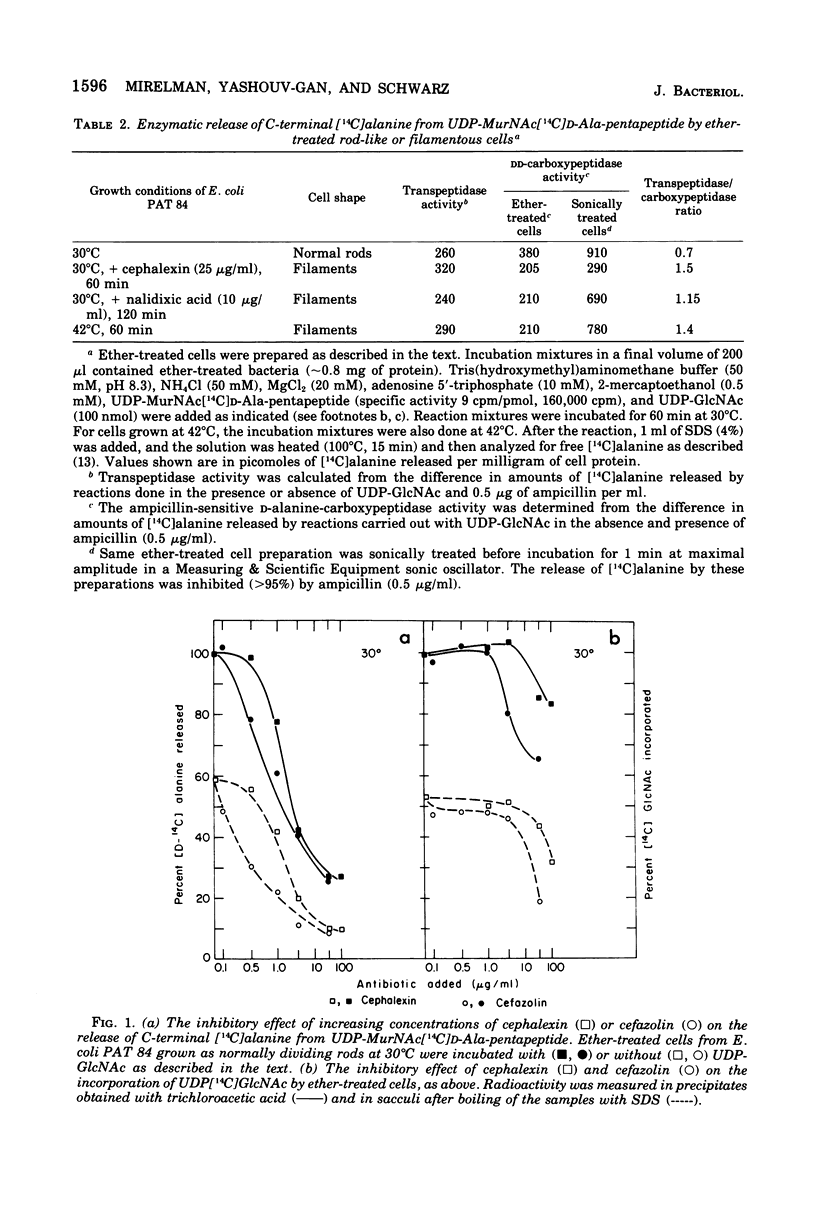
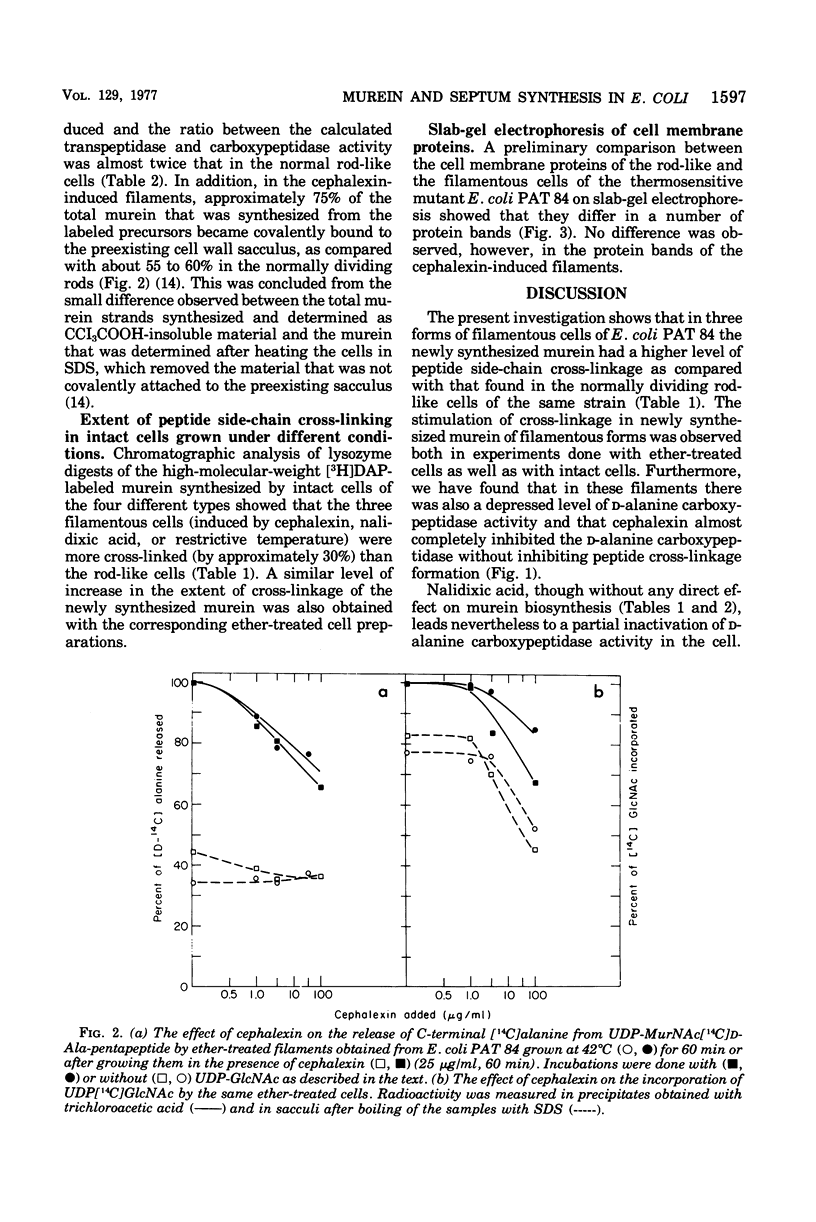
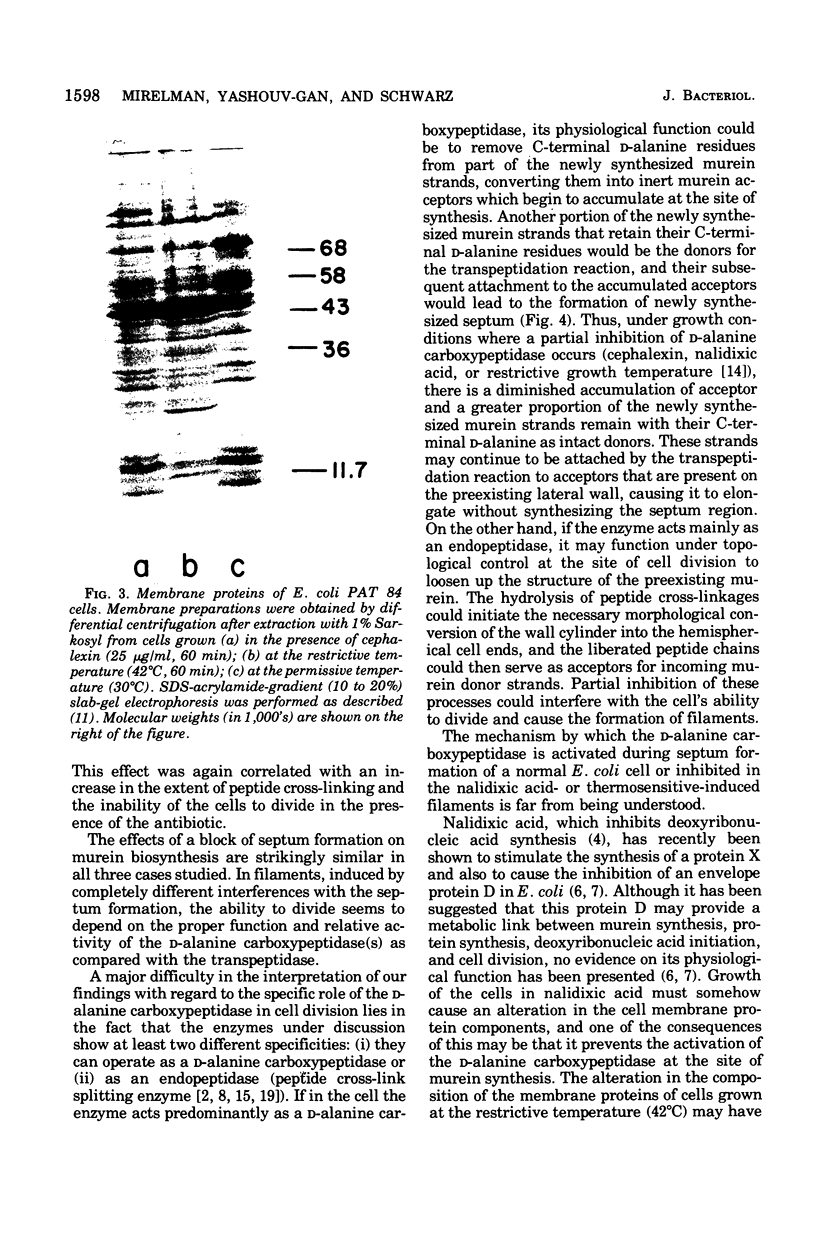
Both the beta-lactam antibiotic, cephalexin, and the deoxyribonucleic acid synthesis inhibitor, nalidixic acid, are known to inhibit cell division in Escherichia coli and induce the formation of filaments. The biosynthesis of murein was investigated in these filaments and compared with the murein synthesized by the normally dividing rods of E. coli PAT 84. Differences were found in the extent of peptide side-chain cross-linkage. Filamentous cells had higher extents of cross-linkages in their newly synthesized murein. Quantitative analyses of the D-alanine carboxypeptidase and transpeptidase reactions in the different cells revealed that the carboxypeptidase activity of the filamentous cells was partially inhibited. These results were similar to those previously found with filaments that were obtained after growth of the thermosensitive division mutant at its restrictive temperature. We conclude that the formation of new cell ends (septa) depends on the proper balance between the activities of the D-alanine carboxypeptidase that regulates the availability of precursor doners and the transpeptidase, which catalyzes cross-linking and attachment of newly synthesized murein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bricas E., Dezélée P. Sur l'idenité de la mucoendopeptidase et de la carboxy-peptidase I d'Escherichia coli, eneymes hydrolysant des liaisons de configuration D-D et inhibées par la pénicilline. C R Acad Sci Hebd Seances Acad Sci D. 1969;269(3):390–393. [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Comparison of the responses of Escherichia coli and proteus mirabilis to seven beta-lactam antibodies. J Infect Dis. 1973 Aug;128(2):211–222. doi: 10.1093/infdis/128.2.211. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., James R., Paradee A. B. Evidence of the involvement of an outer membrane protein in DNA initiation. J Biol Chem. 1976 Jun 10;251(11):3470–3479. [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lect. 1968 1969;64:179–213. [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]