Abstract
Immune mechanisms contribute to cerebral ischemic injury. Therapeutic immunosuppressive options are limited due to systemic side effects. We attempted to achieve immunosuppression in the brain through oral tolerance to myelin basic protein (MBP). Lewis rats were fed low-dose bovine MBP or ovalbumin (1 mg, five times) before 3 h of middle cerebral artery occlusion (MCAO). A third group of animals was sensitized to MBP but did not survive the post-stroke period. Infarct size at 24 and 96 h after ischemia was significantly less in tolerized animals. Tolerance to MBP was confirmed in vivo by a decrease in delayed-type hypersensitivity to MBP. Systemic immune responses, characterized in vitro by spleen cell proliferation to Con A, lipopolysaccharide, and MBP, again confirmed antigen-specific immunologic tolerance. Immunohistochemistry revealed transforming growth factor β1 production by T cells in the brains of tolerized but not control animals. Systemic transforming growth factor β1 levels were equivalent in both groups. Corticosterone levels 24 h after surgery were elevated in all sham-operated animals and ischemic control animals but not in ischemic tolerized animals. These results demonstrate that antigen-specific modulation of the immune response decreases infarct size after focal cerebral ischemia and that sensitization to the same antigen may actually worsen outcome.
Inflammation plays a role in propagating cerebral ischemic injury and, consequently, immunosuppressive agents improve outcome in experimental models of stroke (1–4). Most available immunosuppressive agents, however, have systemic side effects that would limit therapeutic use in stroke. We attempted to control inflammation in the brain by inducing oral tolerance to the central nervous system (CNS) antigen, myelin basic protein (MBP). Oral tolerance is a well-established model whereby immunologic tolerance is induced to a specific antigen through feeding of that antigen (5, 6). The nature of the tolerance depends upon the amount and schedule of antigen feeding. Clonal deletion of antigen-reactive T cells occurs after a single feeding of a very high dose of antigen (7, 8), and active tolerance occurs after repetitive low-dose feeding of the antigen (9–13). Upon restimulation with the appropriate antigen, T cells in animals tolerized with a low-dose regimen secrete cytokines such as transforming growth factor β1 (TGF-β1), interleukin (IL) 4, and IL-10 (9–11), which suppress cell-mediated, or Th1, immune responses. As a result, the immune response is deviated toward a humoral, or Th2, response. Although activation of these T cells is antigen-specific, the immunomodulatory cytokines secreted in response to activation have nonspecific effects. Thus, immunosuppression will occur wherever antigen is present, regardless of whether or not that antigen prompted the initial immune response. This phenomenon, known as bystander suppression (6), leads to relatively organ-specific immunosuppression (14).
Cerebral injury leads to breakdown of the blood–brain barrier (BBB) (15–17), exposing CNS antigens to the peripheral circulation (18–23) and allowing the peripheral circulation access to the brain. Immune responses to these antigens after stroke are not well characterized and may contribute to the pathogenesis of ischemic injury. The literature focuses on the contribution of neutrophils to cerebral ischemic injury, but T cells and natural killer cells are also present in the brain within 24 h after stroke (24). Active immunologic tolerance to CNS antigens, such as MBP, theoretically could decrease cerebral inflammation and improve outcome after stroke through bystander suppression. We hypothesized that rats orally tolerized to MBP would have smaller infarcts after transient middle cerebral artery occlusion (MCAO) than control animals. Conversely, animals sensitized to MBP should have a more robust inflammatory response, and consequently, larger infarcts.
MATERIALS AND METHODS
Animals.
Male Lewis rats (6–10 weeks old) were tolerized to bovine MBP (OT-MBP, n = 23) by repetitive gavage with 1 mg of protein in 0.5 ml of PBS every 2–3 days for 2 weeks (a total of five feedings). A second group of animals was fed ovalbumin (OVA; 1 mg/0.5 ml of PBS) on a similar schedule (control, n = 38). A third group of animals was sensitized to MBP (Sens-MBP, n = 6) by injection of guinea pig MBP (50 μg of MBP in 50 μl of PBS mixed with an equal amount of complete Freund’s adjuvant) into the hind footpad. Bovine MBP, OVA, and complete Freund’s adjuvant were purchased from Sigma. Guinea pig MBP was a gift from Laura Quigley (National Institute of Neurological Disorders and Stroke, Bethesda). Two days after the last feeding (OT-MBP and control) or 6 days after immunization (Sens-MBP), the middle cerebral artery was occluded under halothane anesthesia (2%) via a 4.0-monofilament suture inserted into the internal carotid artery and advanced 17–20 mm (25). In sham-operated animals, the suture was inserted into the carotid artery but not advanced (OT-MBP, n = 11; control, n = 9). The average duration of surgery and anesthetic administration was 15 min. Body temperature was continuously monitored during surgery and maintained at 37–38°C with a thermostatically controlled warming blanket, but animals were allowed to thermoregulate thereafter. Three hours after MCAO, reperfusion was established by withdrawing the suture under brief anesthesia. Sham-operated animals underwent the same brief period of anesthesia. Animals were sacrificed by cardiac puncture and decapitation under halothane anesthesia 24 h (OT-MBP, control) or 4 days (OT-MBP, control, and Sens-MBP) after ischemia. Immunologic parameters were referenced to a group of naive animals with no prior manipulations (n = 11). Blood was collected into heparinized syringes at sacrifice and plasma samples stored at −70°C until needed.
Infarct Size.
In the first experiments, animals were sacrificed 24 h after ischemia. Brains were sectioned coronally and incubated in 2% triphenyl tetrazolium chloride (Sigma) at 38°C for 30 min. In the second experiments, animals were sacrificed 4 days after ischemia, and brains were frozen, sectioned coronally at 16-μm intervals (because of marked necrotic injury, tissue integrity could not be maintained with thinner sections), and stained with cresyl violet. Infarct size was determined by using a digitized imaging system (NIH Image). Sections stained with triphenyl tetrazolium chloride were analyzed at five predetermined levels (bregma +1.00, −0.40, −1.80, −3.20, and −4.40); sections stained with cresyl violet were analyzed at six predetermined levels (bregma +2.40, +1.00, −0.40, −1.80, −3.20, and −4.40). Both direct and indirect measurements [to correct for the presence of edema (26)] of infarct size were made.
In Vivo Immune Responses.
Delayed-type hypersensitivity responses were assessed to confirm the efficacy of the tolerizing regimen. Animals were tolerized to MBP (n = 4) or ovalbumin (OVA, n = 3). Control animals (n = 4) were not handled. All animals then were immunized with bovine MBP (50 μg of MBP in 50 μl of PBS mixed with 50 μl of complete Freund’s adjuvant and injected into the hind footpad) and then rechallenged with bovine MBP (50 μg in 50 μl of PBS) injected into the ear 15 days later. Change in ear thickness, expressed in millimeters, was measured with microcalipers (Mitutoyo, Japan) 48 h later by an observer blinded to treatment.
In Vitro Immune Responses.
Spleens were removed aseptically, single-cell suspensions were prepared, and the total number of cells was determined. Splenocyte proliferation was assessed 24 h or 4 days after ischemia. Cells were suspended in RPMI 1640 medium (supplemented with 10% fetal calf serum, 0.05 M 2-mercaptoethanol, 1% sodium pyruvate, 1% nonessential amino acids, 1% l-glutamine, 1% penicillin-streptomycin, and 1 M Hepes) and cultured in 96-well microtiter plates (1.5 × 104 cells per well) in a volume of 0.2 ml per well. All cell culture reagents were purchased from GIBCO/Life Technologies. Cells were cultured with the T cell mitogen Con A (4 μg/ml), the B cell mitogen lipopolysaccharide (Escherichia coli, serotype 0111:B4; 0.25 μg/ml), or bovine MBP (25 μg/ml) for 144 h. Mitogens were purchased from Sigma. Proliferation was assessed by pulse-labeling cells with [methyl-3H]thymidine at 1 μCi per well (specific activity 25 Ci/mmol; Amersham; 1 Ci = 37 GBq) for the final 18 h of culture and harvesting onto glass fiber filters (Skatron, Sterling, VA) by using a multichannel harvesting device (Mash II; Skatron). Incorporation of [methyl-3H]thymidine was measured by liquid scintillation spectroscopy and results were expressed as stimulation indices (SI). The SI is calculated by dividing the cpm of the mitogen- or antigen-stimulated cells by the cpm of the cells incubated in medium alone. All cultures were performed in quadruplicate.
TGF-β1 Assay.
Plasma TGF-β1 levels were determined with an enzyme-linked immunoassay kit for human TGF-β1 (R & D Systems) that cross-reacts with the rat protein. Values are expressed in ng/ml with the lower limit of detection equal to 0.005 ng/ml.
Steroid Assay.
Corticosterone levels were assessed by standard radioimmunoassay (ICN). Detection limits were approximately 25 ng/ml to 1000 ng/ml.
Histology and Immunohistochemistry.
Histology was performed on animals sacrificed 4 days after MCAO or sham surgery. Brains were frozen, sectioned at 16 μm, and stored at −70°C until staining. Sections were fixed in 4% paraformaldehyde and incubated with the following antibodies: rabbit polyclonal anti-rat IL-1β (Cytokine Sciences, Boston; 1:100 dilution), mouse monoclonal anti-porcine glial fibrillary acidic protein (Sigma; 1:1,000 dilution), rabbit polyclonal anti-rat TGF-β1 (LC1–30-1; provided by Kathleen Flanders, National Cancer Institute; 1:300 dilution) (27), mouse monoclonal anti-rat pan T cell marker OX-52 (Biosource; 1:10 dilution), and a mixture of mouse monoclonal antibodies to several different rat neurofilament markers, SMI 311 (Sternberger Monoclonals, Baltimore, 1:1,000 dilution). Antibody binding sites were visualized with fluorescein isothiocyanate and Texas Red. Negative controls were performed by replacing the primary antibody with nonimmune Ig.
Statistics.
Data are expressed as the mean ± SEM. Statistical significance was determined by repeated-measures ANOVA (infarct size) or by one-way ANOVA with post-hoc Dunnett’s test (corticosterone levels and immune responses). All tests were two-tailed and significance was set at P < 0.05.
RESULTS
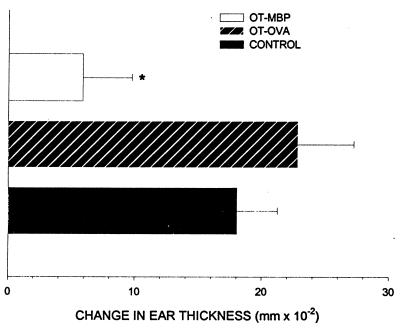
Animals fed bovine MBP before immunization with bovine MBP had a significant decrease in the delayed-type hypersensitivity response, as determined by increase in ear thickness (0.059 ± 0.039 mm; P < 0.05), compared with animals fed OVA (0.228 mm ± 0.044) or control animals (0.180 ± 0.032 mm; Fig. 1).
Figure 1.
Delayed-type hypersensitivity reactions. The degree of ear swelling in MBP-immunized animals 48 h after rechallenge with MBP. Animals were orally tolerized to MBP (OT-MBP), orally tolerized to OVA, an irrelevant antigen (OT-OVA), or were not tolerized at all (control). ∗, significant difference from control and OT-OVA at P < 0.05 as determined by one way ANOVA with post-hoc Dunnett’s test.
All animals undergoing MCAO demonstrated clinical evidence of stroke and became febrile during ischemia (OT-MBP = 38.6 ± 0.13°C; control = 38.6 ± 0.06°C; Sens-MBP = 38.3 ± 0.19°C). A further increase in temperature occurred during the first 3 h of reperfusion (OT-MBP = 39.0 ± 0.08°C; control = 39.3 ± 0.10°C; Sens-MBP = 38.6 ± 0.24°C). OT-MBP temperatures returned to baseline by 24 h after MCAO (37.7 ± 0.17°C) but control temperatures (38.2 ± 0.19°C) remained slightly elevated. Only two Sens-MBP animals were alive at 24 h; their mean (±SEM) temperature was 38.0 ± 0.15°C. There were no significant differences among OT-MBP, control, or Sens-MBP temperatures at any time.
In the first experiments (3 h of ischemia and 21 h of reperfusion), there was a nonsignificant trend toward decreased mortality in OT-MBP (12.5%, n = 8) as compared with control (46.7%, n = 15) animals. In the second experiments (3 h of ischemia and 4 days of reperfusion), there was a 100% 4-day mortality among animals sensitized to guinea pig MBP (Sens-MBP, n = 6). Limited necropsy revealed herniation as the etiology of death. There was a trend toward decreased mortality at 4 days in OT-MBP (33.3%, n = 15) as compared with control (56.5%, n = 23) animals, but the differences in 4-day mortality among Sens-MBP, OT-MBP, and control animals were not statistically significant. The mortality for control animals undergoing MCAO is not different for those that have or have not been fed OVA (data not shown).
Infarct Size.
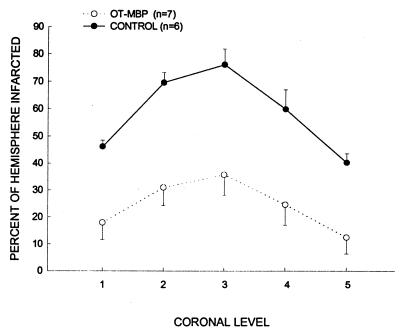
Infarcts had the features of pan necrosis. Infarct size 24 h after ischemia was significantly less in OT-MBP as compared with control animals at each of the five levels assessed (Fig. 2). With the effect of edema eliminated, differences are less marked but still significant (data not shown). Infarct size 96 h after ischemia, corrected for the presence of edema, was again significantly less in OT-MBP animals (Fig. 3).
Figure 2.
Measurement of infarct size at five predetermined coronal levels 24 h after ischemia in OT-MBP (n = 7) and control (n = 6) animals as detected by triphenyl tetrazolium chloride staining. Groups differ from each other as determined by repeated measures ANOVA (P < 0.05).
Figure 3.
Infarct size, measured on cresyl violet-stained sections, 96 h after MCAO. Infarct size, corrected for the presence of edema, is significantly less in OT-MBP animals (n = 8) as compared with control animals (n = 9). Groups differ from each other as determined by repeated measures ANOVA (P < 0.05).
In Vitro Immune Responses.
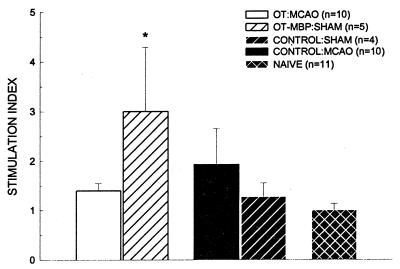
Compared with naive animals (90.2 ± 15.4), there was a nonsignificant trend in ischemic animals for decreased splenocyte proliferation to Con A at both 24 and 96 h. There were no significant differences among the experimental groups at either time point (OT-MBP = 64.4 ± 13.0 and 54.8 ± 11.1; control = 53.1 ± 8.06 and 79.5 ± 14.0; 24 and 96 h, respectively). Similarly, there were no significant differences in proliferation to lipopolysaccharide at 24 (1.53 ± 0.84 and 1.89 ± 0.45) or 96 (7.86 ± 2.32 and 7.02 ± 2.61) h among OT-MBP or control animals, respectively. Sham-operated OT-MBP animals showed a significant increase in proliferation to MBP (3.00 ± 1.29; P < 0.05) 96 h after surgery, compared with naive animals (1.00 ± 0.13) that was suppressed with ischemia (1.40 ± 0.15). MBP induced splenocyte proliferation in ischemic control (1.93 ± 0.72) and sham-operated control (1.27 ± 0.29) animals was no greater than in naive animals (Fig. 4).
Figure 4.
Stimulation indices to MBP in animals 4 days after MCAO or sham surgery (OT-MBP, n = 5; control, n = 4). Experimental groups are compared with naive animals (n = 11) and significance set at P < 0.05 (∗) as determined by one-way ANOVA with post-hoc Dunnett’s test.
TGF-β1 Levels.
There were no significant differences in plasma TGF-β1 levels among OT-MBP (24.2 ± 8.5 ng/ml, n = 6), control (31.3 ± 5.74 ng/ml, n = 9), and naive (16.9 ± 2.57 ng/ml, n = 7) animals 24 h after MCAO. TGF-β1 levels decreased 96 h after MCAO in both OT-MBP (18.8 ± 5.53 ng/ml, n = 10) and control (12.9 ± 2.57 ng/ml, n = 10) animals, but only in the latter group did the mean fall below that of naive animals. TGF-β1 levels in sham-operated animals did not differ from their ischemic counterparts or from naive animals at either time point (data not shown).
Steroid Levels.
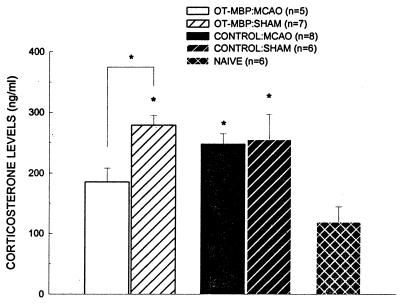
Plasma corticosterone levels were significantly elevated 24 h after surgery in ischemic control animals (247.5 ± 17.27 ng/ml) and both sham-operated OT-MBP (278.8 ± 16.39 ng/ml) and control (254.7 ± 42.09 ng/ml) animals as compared with naive animals (118.7 ± 25.46 ng/ml) (P < 0.05 for all). Only ischemic OT-MBP animals (185.3 ± 22.84 ng/ml) failed to evidence a rise in corticosterone (Fig. 5). Four days after surgery, corticosterone levels in ischemic OT-MBP (201.7 ± 14.4 ng/ml) and control (218.8 ± 20.75 ng/ml) animals, but not in sham-operated OT-MBP (292.2 ± 64.77 ng/ml) or control (253.5 ± 74.70 ng/ml) animals, were still significantly greater than in naive animals (P < 0.05).
Figure 5.
Corticosterone levels 24 h after MCAO (OT-MBP, n = 5; control, n = 8) or sham surgery (OT-MBP, n = 7; control, n = 6). Experimental groups are compared with naive animals (n = 6) and significance set at P < 0.05 (∗) as determined by one way ANOVA with post-hoc Dunnett’s test.
Immunohistochemistry.
No labeling was seen with negative controls. Robust TGF-β1 labeling, which was largely neuronal, was seen 4 days after ischemia in OT-MBP and control animals. Astrocytes did not evidence TGF-β1 production. T cells were present in all ischemic animals. They were primarily intravascular in control animals and they were more numerous, intravascular, and frequently intraparenchymal in OT-MBP animals. Most T cells stained with both TGF-β1 and OX-52 in OT-MBP animals but very few evidenced double labeling in control animals (Figs. 6). IL-1β was present within astrocytes of the ischemic penumbra in both groups. No evidence of T cells or IL-1β was seen in sham-operated or naive animals. Neuronal TGF-β1 was seen in all animals, although the magnitude was greatest in those with ischemia.
Figure 6.
(A) T cells in orally tolerized animals (OT-MBP) are immunopositive for both OX-52, a pan T cell marker, and TGF-β1. OX-52 is detected with fluorescein isothiocyanate and TGF-β1 is detected with Texas red. Cells immunopositive for both markers fluoresce yellow (arrowhead). (B) T cells in control animals are not immunopositive for TGF-β1. There are distinct populations of OX-52-positive cells (fluorescein isothiocyanate, straight arrow) and TGF-β1-positive cells (Texas Red, arrowhead).
DISCUSSION
Inflammation contributes to cerebral injury in stroke (28, 29). Outcome after cerebral ischemia is improved by anti-inflammatory strategies, such as depleting the circulating pool of leukocytes (30, 31), inhibiting leukocyte function (32), and blocking leukocyte adhesion (33, 34), and by immunosuppressive drugs, such as high-dose steroids (35), cyclosporine A (1, 2), and FK506 (3, 4). At present, however, there are no interventions that limit the CNS inflammatory response without also affecting systemic responses. We attempted to control inflammation in an organ-specific fashion by inducing oral tolerance to the CNS antigen MBP. By using a model of low-dose tolerance, we demonstrate that MBP-specific modulation of the immune response results in decreased infarct size at both 24 and 96 h after stroke. Because the presence of water in tissue overestimates true infarct size, the contribution of edema must be eliminated for an accurate measurement. Nonetheless, we included the uncorrected measure at 24 h because edema is an important marker of secondary injury. Cerebral edema can impair collateral blood flow, worsen ischemia, and ultimately lead to cerebral herniation and death.
Oral administration of an antigen results in deviation of the lymphocyte response from a Th1 to a Th2- (36) or Th3-like (9, 13) response. Th1 responses are characterized by secretion of proinflammatory cytokines such as IL-1β, tumor necrosis factor β, IL-2, IL-12, and interferon γ, and Th2 responses are characterized by secretion of cytokines such as IL-4, IL-5, IL-6, IL-10, and IL-13, which suppress cell-mediated responses and favor the development of humoral responses (37–39). TGF-β1-producing cells induced by oral administration of antigen have been coined Th3 cells (9, 13). Aspects of oral tolerance unique to the CNS are best described for experimental allergic encephalomyelitis (EAE). EAE occurs after immunization of susceptible animals with MBP in adjuvant. Approximately 8–10 days after immunization the permeability of the BBB is altered (40) and T cells infiltrate the brain (41). By day 10–15, animals develop pathologic and clinical signs of EAE (42, 43), including mild to severe hind-limb paresis, weight loss, and death. Feeding animals MBP prior to immunization can prevent EAE. This occurs by an active process whereby T cells primed to MBP during feeding generate a Th2 or Th3-like response upon re-encounter with the antigen (6, 9–11, 36, 44). Of the cytokines secreted by these T cells, TGF-β1 is crucial for preventing EAE (10, 11, 14, 44–46). And even though TGF-β1 secretion is stimulated in an antigen-specific fashion, TGF-β1 itself has no antigen specificity. Thus, immunosuppression occurs wherever antigen is present (6).
Because the BBB is disrupted in stroke (15–17), immune cells gain access to brain and relatively sequestered CNS antigens are exposed to the peripheral circulation. The breakdown of the BBB leads to rapid acquisition of MBP-reactive T cell clones and Igs in stroke patients (47–49) but does not lead to autoimmune encephalitis. Why these autoreactive T cells do not cause disease is unclear but may be related to the lack of appropriate costimulatory molecules in the brains of stroke patients (50). Accordingly, the use of MBP to manipulate the immune response does not imply that immunologic responses to MBP are pathologic. MBP was chosen as the antigen in this study because serum levels of MBP are elevated after stroke (20), precise feeding regimens for inducing tolerance to MBP are documented (9, 10, 51), and the mechanisms of this tolerance are well studied (9, 10). Any CNS-specific antigen exposed to the peripheral circulation after breakdown of the BBB could theoretically alter the local immune response after stroke inasmuch as activated antigen-specific T cells would enter the CNS circulation and secrete immunoregulatory cytokines, such as TGF-β1, upon encountering antigen.
Animals sensitized to MBP did not survive MCAO. This likely occurred because, as a result of immunization, immunocompetent MBP-reactive T cells were present and able to mount an immune response upon breakdown of the BBB. Animals underwent MCAO 6 days after immunization with MBP because this is when T cells are beginning to accumulate within the cerebral vasculature but no clinical or pathologic signs of disease are present. These primed T cells elaborate proinflammatory cytokines that produce the pathology seen in EAE (10, 52–54) and also worsen outcome in stroke (55–58). This, then, may represent a situation where appropriate costimulation is present in the brain as a result of immunization (59), and when antigen is presented to the autoreactive T cells after stroke, an overwhelming cerebral inflammatory response occurs leading to edema, herniation, and death. This is also consistent with the observation that focal brain lesions enhance the activity of EAE (60).
There were no differences in mitogen-induced splenocyte proliferation among experimental and naive animals, suggesting that the neuroprotection conferred by oral tolerance was not due to generalized immunosuppression. The antigen specificity of tolerance was documented by abrogation of the delayed-type hypersensitivity response to MBP. In addition, sham-operated tolerized animals had increased MBP responsiveness that was suppressed by ischemia. This pattern of in vitro responses has been documented (61) and suggests that there is antigen-specific priming of T cells with feeding that requires antigen reexposure to induce the immunosuppressive phenotype.
Mortality was not a prespecified outcome in this study, but because of the high mortality in control animals after MCAO, corticosterone levels were assessed to determine the magnitude of the stress response to repeated handling and oral gavage. In comparison to other strains, Lewis rats have a blunted response of the hypothalamic–pituitary–adrenal axis to stress (62, 63), making them more prone to inflammatory and immunologic diseases, and, in part accounting for their susceptibility to EAE (64, 65). Plasma corticosterone levels were not elevated in OT-MBP animals 24 h after stroke as they were in control animals. But because corticosterone levels were elevated in sham-operated OT-MBP animals, cerebral ischemia in these animals may have affected corticosterone production. Hence, the effects of tolerance were not strictly organ-specific since systemic corticosterone levels were altered. How MCAO attenuated corticosterone responses in tolerized animals was not assessed, but it is possible that there were transient differences in systemic TGF-β1 levels that were not appreciated because of its short half-life (66). TGF-β1 inhibits steroid production at the level of the adrenal medulla (67). Furthermore, the effect of central TGF-β1 on steroid production has not been systematically studied, so it is possible that central TGF-β1 could also attenuate the steroid response.
Although we have shown that antigen-specific tolerance decreases infarct size in the Lewis rat model, the mechanism of neuroprotection has not been fully addressed. Exogenous administration of TGF-β1 limits injury and improves outcome in several models of cerebral ischemia (68, 69). This may be related to its immunosuppressive properties, but TGF-β1 also has direct effects on neuronal survival (70, 71). There is rapid up-regulation of TGF-β1 mRNA after cerebral ischemia (72, 73), suggesting an endogenous role for TGF-β1 in limiting cerebral injury. Even nanogram quantities of TGF-β1, when injected directly into ischemic brain tissue, can salvage neurons (74). In the present model, systemic levels of TGF-β1 were not altered by tolerance, yet there was an apparent difference in TGF-β1 production within the brains of tolerized and control animals as evidenced by double-label immunohistochemistry 4 days after MCAO. Exact quantification of TGF-β1 in the brain, or administration of TGF-β1 neutralizing antibodies, would help confirm its role in the protective response. And because even physiologic levels of steroids worsen outcome in stroke (75), preventing the initial systemic increase in corticosterone after ischemia in tolerized animals may also have been protective.
Finally, the populations of lymphocytes, be they systemic or resident brain T cells, responsible for the protective effect seen in this model is unclear. If systemic lymphocytes are important for protection, preventing lymphocyte trafficking into the brain with antiadhesion molecules after ischemia should abrogate the protective effect. Additionally, if systemic T cells are responsible, adoptive transfer of lymphocytes from tolerized animals to naive recipients undergoing ischemia should confer protection. These experiments also might be repeated in another strain of rat where the immune system behaves differently. Fischer rats, for example, have a robust hypothalamic–pituitary–adrenal response to stress (62, 63) and are resistant to inflammatory disease (65, 76). Comparison of outcome in Lewis and Fischer rats after MCAO may help to address the relative contribution of the immune system and inflammation to ischemic cerebral injury and further delineate the mechanisms and utility of immune deviation therapy in stroke.
CONCLUSIONS
Because of the breakdown of the BBB after stroke, one can capitalize on the unique exposure of relatively sequestered CNS antigens to the peripheral circulation. We have shown that MBP-specific modulation of the immune response can decrease infarct size after transient MCAO in Lewis rats. Histology suggests that this is due to a Th2- or Th3-like response in the brains of tolerized animals. Furthermore, enhancing immune responses to CNS antigens, as was done in sensitized animals, may worsen outcome after stroke.
Oral tolerance effectively initiates antigen-specific and, through the bystander effect (6), organ-specific immunosuppression (14). This phenomenon allows for its use in the treatment of autoimmune disease. Oral tolerance is most easily induced in mice and rats, but other species can also be tolerized, and have been, to treat autoimmune arthritis (77), uveitis (78), myasthenia gravis (79, 80), diabetes (81), colitis (82), and thyroiditis (83), as well as EAE and multiple sclerosis (84). Once induced, tolerance is sustained for months (85). Humans can be orally tolerized, and administration of MBP to humans has proven to be safe (84). It is therefore conceivable that patients with cerebrovascular disease, or risk factors for cerebrovascular disease, be prophylactically tolerized to CNS antigens so that in the event of stroke, specific immunosuppression is in place. Immune deviation also occurs with inhalation of aerosolized antigens (77, 86), and since the effects of a fed or inhaled protein on T cell function are quite rapid (87), it would be possible to treat acute stroke patients with emergent administration of MBP. The optimism for therapeutic applications, however, is tempered by recent reports documenting induction of cytotoxic T lymphocyte responses in mice (88) and pathogenic autoantibody production in marmosets (89) after immune deviation therapy. Thus, the long-term effects of this model need to be assessed before it can be advocated for clinical use in stroke.
ABBREVIATIONS
- MBP
myelin basic protein
- BBB
blood–brain barrier
- MCAO
middle cerebral artery occlusion
- TGF
transforming growth factor
- IL
interleukin
- CNS
central nervous system
- EAE
experimental allergic encephalomyelitis
References
- 1.Kondo Y, Ogawa N, Asanuma M, Nishibayashi S, Iwata E, Mori A. Neurosci Res. 1995;22:123–127. doi: 10.1016/0168-0102(95)00878-w. [DOI] [PubMed] [Google Scholar]
- 2.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Stroke. 1995;26:1415–1422. doi: 10.1161/01.str.26.8.1415. [DOI] [PubMed] [Google Scholar]
- 3.Sharkey J, Butcher S P. Nature (London) 1994;371:336–339. doi: 10.1038/371336a0. [DOI] [PubMed] [Google Scholar]
- 4.Tokime T, Nozaki K, Kikuchi H. Neurosci Lett. 1996;206:81–84. doi: 10.1016/s0304-3940(96)12438-1. [DOI] [PubMed] [Google Scholar]
- 5.Khoury S J, Lider O, Al-Sabbagh A, Weiner H L. Cell Immunol. 1990;131:301–310. doi: 10.1016/0008-8749(90)90256-q. [DOI] [PubMed] [Google Scholar]
- 6.Miller A, Lider O, Weiner H L. J Exp Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Inobe J-i, Marks R, Gonnella P, Kuchroo V K, Weiner H L. Nature (London) 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 8.Whitacre C C, Gienapp I E, Orosz C G, Bitar D M. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo V K, Inobe J-i, Hafler D A, Weiner H L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.Khoury S J, Hancock W W, Weiner H L. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller A, Lider O, Roberts A, Sporn M B, Weiner H L. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Inobe J-i, Kuchroo V K, Baron J L, Janeway C A, Weiner H L. Proc Natl Acad Sci USA. 1996;93:388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fakaura H, Kent S, Pietrusewicz M, Khoury S, Weiner H, Hafler D. J Clin Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santambrogio L, Hochwald G M, Saxena B, Leu C-H, Martz J E, Carlino J A, Ruddle N H, Palladino M A, Gold L I, Thorbecke G J. J Immunol. 1993;151:1116–1127. [PubMed] [Google Scholar]
- 15.Cole D J, Matsumura J S, Drummond J C, Schultz R L, Wong M H. Acta Neuropathol. 1991;82:266–273. doi: 10.1007/BF00308811. [DOI] [PubMed] [Google Scholar]
- 16.Dobbin J, Crockard H A, Ross-Russell R. J Cereb Blood Flow Metab. 1989;9:71–78. doi: 10.1038/jcbfm.1989.10. [DOI] [PubMed] [Google Scholar]
- 17.Nagahiro S, Goto S, Korematsu K, Sumi M, Takahashi M, Ushio Y. Brain Res. 1994;633:305–311. doi: 10.1016/0006-8993(94)91553-9. [DOI] [PubMed] [Google Scholar]
- 18.Horn M, Seger F, Schote W. Stroke. 1995;26:290–297. doi: 10.1161/01.str.26.2.290. [DOI] [PubMed] [Google Scholar]
- 19.Skogseid I M, Nordby H K, Urdal P, Paus E, Lilleaas F. Acta Neurochir. 1992;115:106–111. doi: 10.1007/BF01406367. [DOI] [PubMed] [Google Scholar]
- 20.Palfreyman J W, Johnston R V, Ratcliffe J G, Thomas D G T, Forbes C D. Clin Chim Acta. 1979;92:403–409. doi: 10.1016/0009-8981(79)90220-1. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki Y, Yada K, Morii S, Kitahara T, Ohwada T. Surg Neurol. 1995;43:267–271. doi: 10.1007/978-4-431-68231-8_86. [DOI] [PubMed] [Google Scholar]
- 22.Schaarsmidt H, Prange H W, Reiber H. Stroke. 1994;25:558–565. doi: 10.1161/01.str.25.3.558. [DOI] [PubMed] [Google Scholar]
- 23.Butterworth R J, Wassif W S, Sherwood R A, Gerges A, Poyser K H, Garthwaite J, Peters T J, Bath P M W. Stroke. 1996;27:2064–2068. doi: 10.1161/01.str.27.11.2064. [DOI] [PubMed] [Google Scholar]
- 24.Schroeter M, Jander S, Witte O W, Stoll G. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 25.Zea-Longa E, Weinstein P R, Carlson S, Cummins R. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 26.Swanson R A, Morton M T, Tsao-Wu G, Savalos R A, Davidson C, Sharp F R. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 27.Flanders K C, Thompson N L, Cissel D S, Van Obberghen-Shilling E, Baker C C, Kass M E, Ellingsworth L R, Roberts A B, Sporn M B. J Cell Biol. 1989;108:653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallenbeck J M. Adv Neurol. 1996;71:281–297. [PubMed] [Google Scholar]
- 29.Kochanek P M, Hallenbeck J M. Stroke. 1992;23:1367–1379. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- 30.Dutka A, Kochanek P, Hallenbeck J. Stroke. 1989;20:390–395. doi: 10.1161/01.str.20.3.390. [DOI] [PubMed] [Google Scholar]
- 31.Bednar M M, Raymond S, McAuliffe T, Lodge P A, Gross C E. Stroke. 1991;22:44–50. doi: 10.1161/01.str.22.1.44. [DOI] [PubMed] [Google Scholar]
- 32.Clark W M, Calcagno F A, Gabler W L, Smith J R, Coull B M. Stroke. 1994;25:1411–1415. doi: 10.1161/01.str.25.7.1411. [DOI] [PubMed] [Google Scholar]
- 33.Chopp M, Zhang R L, Chen H, Li Y, Jiang N, Rusche J R. Stroke. 1994;25:869–875. doi: 10.1161/01.str.25.4.869. [DOI] [PubMed] [Google Scholar]
- 34.Mori E, del Zoppo G J, Chambers D, Copeland B R, Arfors K E. Stroke. 1992;23:712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- 35.de Courten-Myers G M, Kleinholz M, Wagner K R, Xi G, Myers R E. Stroke. 1994;25:487–493. doi: 10.1161/01.str.25.2.487. [DOI] [PubMed] [Google Scholar]
- 36.Fishman-Lobell J, Friedman A, Weiner H L. Eur J Immunol. 1994;24:2720–2724. doi: 10.1002/eji.1830241122. [DOI] [PubMed] [Google Scholar]
- 37.Mossman T R, Sad S. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 38.Lucey D, Clerici M, Shearer G. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsson T. Immunol Rev. 1995;144:245–268. doi: 10.1111/j.1600-065x.1995.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 40.Namer I J, Steibel J, Poulet P, Armspach J P, Mauss Y, Chambron J. Magn Reson Med. 1992;24:325–334. doi: 10.1002/mrm.1910240213. [DOI] [PubMed] [Google Scholar]
- 41.Traugott U. Cell Immunol. 1989;119:114–129. doi: 10.1016/0008-8749(89)90228-1. [DOI] [PubMed] [Google Scholar]
- 42.D’Amelio F E, Smith M E, Eng L F. Glia. 1990;3:229–240. doi: 10.1002/glia.440030402. [DOI] [PubMed] [Google Scholar]
- 43.Claudio L, Kress Y, Factor J, Brosnan C F. Am J Pathol. 1990;137:1033–1045. [PMC free article] [PubMed] [Google Scholar]
- 44.Santos L M B, Al-Sabbagh A, Londono A, Weiner H L. Cell Immunol. 1994;157:439–447. doi: 10.1006/cimm.1994.1240. [DOI] [PubMed] [Google Scholar]
- 45.Aroeira L S, Cardillo F, De Albuquerque D A, Vaz N M, Mengel J. Scand J Immunol. 1995;41:319–323. doi: 10.1111/j.1365-3083.1995.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 46.Johns L D, Sriram S. J Neuroimmunol. 1993;47:1–8. doi: 10.1016/0165-5728(93)90278-7. [DOI] [PubMed] [Google Scholar]
- 47.Alvord E C, Hsu P C, Thron R. Arch Neurol. 1974;30:296–299. doi: 10.1001/archneur.1974.00490340024004. [DOI] [PubMed] [Google Scholar]
- 48.Youngchaiyud U, Coates A S, Whittingham S, Mackay I R. Aust N Z J Med. 1974;4:535–538. doi: 10.1111/j.1445-5994.1974.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 49.Hashim G A, Lee D H, Pierce J C, Braun C W, Fitzpatrick H F. Neurochem Res. 1978;3:37–48. doi: 10.1007/BF00964359. [DOI] [PubMed] [Google Scholar]
- 50.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe M, Cuzner M, Hafler D. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman A, Weiner H L. Proc Natl Acad Sci USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauer J, Berkenbosch F, Van Dam A M, Dijstra C D. J Neuroimmunol. 1993;48:189–198. doi: 10.1016/0165-5728(93)90053-2. [DOI] [PubMed] [Google Scholar]
- 53.Villarroya H, Violleau K, Younes-Chennoufi A B, Baumann N. J Neuroimmunol. 1996;64:55–61. doi: 10.1016/0165-5728(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 54.Issazadeh S, Ljungdahl A, Hojeborg B, Mustafa M, Olsson T. J Neuroimmunol. 1995;61:205–212. doi: 10.1016/0165-5728(95)00100-g. [DOI] [PubMed] [Google Scholar]
- 55.Nawashiro H, Martin D, Hallenbeck J M. J Cereb Blood Flow Metab. 1997;17:229–232. doi: 10.1097/00004647-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Martin D, Chinookoswong N, Miller G. Exp Neurol. 1994;130:362–367. doi: 10.1006/exnr.1994.1215. [DOI] [PubMed] [Google Scholar]
- 57.Garcia J H, Liu K-F, Relton J K. Am J Pathol. 1995;147:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- 58.Barone F C, Arvin B, White R, Miller A, Webb C, Willette R, Lysko P, Feuerstein G. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 59.Racke M K, Scott D E, Quigley L, Gray G S, Abe R, June C H, Perrin P J. J Clin Invest. 1995;96:2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips M, Weller R, Kida S, Iannotti F. Neuropathol Appl Neurobiol. 1995;21:189–200. doi: 10.1111/j.1365-2990.1995.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 61.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson C O. J Immunol. 1994;152:4663–4670. [PubMed] [Google Scholar]
- 62.Sternberg E, Glowa J, Smith M, Calogero A, Listwak S, Aksentijevich S, Chrousos G, Wilder R, Gold P. Brain Res. 1992;570:54–60. doi: 10.1016/0006-8993(92)90563-o. [DOI] [PubMed] [Google Scholar]
- 63.Grota L, Bienen T, Felten D. J Neuroimmunol. 1997;74:95–101. doi: 10.1016/s0165-5728(96)00209-3. [DOI] [PubMed] [Google Scholar]
- 64.Mason D, MacPhee I, Antoni F. Immunology. 1990;70:1–5. [PMC free article] [PubMed] [Google Scholar]
- 65.Griffin A C, Whitacre C C. J Neuroimmunol. 1991;35:53–64. doi: 10.1016/0165-5728(91)90161-y. [DOI] [PubMed] [Google Scholar]
- 66.Coffey R J, Kost L, Lyons R, Moses H, LaRusso N. J Clin Invest. 1987;80:750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rainey W E, Naville D, Saez J M, Carr B R, Byrd W, Magness R R, Mason J I. Endocrinology. 1990;127:1910–1915. doi: 10.1210/endo-127-4-1910. [DOI] [PubMed] [Google Scholar]
- 68.Gross C E, Bednar M M, Howard D B, Sporn M B. Stroke. 1993;24:558–562. doi: 10.1161/01.str.24.4.558. [DOI] [PubMed] [Google Scholar]
- 69.McNeill H, Williams C, Guan J, Dragunow M, Lawlor P, Sirimanne E, Nikolics K, Gluckman P. NeuroReport. 1994;5:901–904. doi: 10.1097/00001756-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 70.Henrich-Noack, P., Prehn, J. H. & Krieglstein, J. (1994) J. Neural Transm., Suppl. 43, 33–45. [PubMed]
- 71.Prehn J H M, Backhaub C, Krieglstein J. J Cereb Blood Flow Metab. 1993;13:521–525. doi: 10.1038/jcbfm.1993.67. [DOI] [PubMed] [Google Scholar]
- 72.Lehrmann E, Kiefer R, Finsen B, Diemer N H, Zimmer J, Hartung H-P. Exp Neurol. 1995;131:114–123. doi: 10.1016/0014-4886(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 73.Krupinski J, Kumar P, Kumar S, Kaluza J. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- 74.Henrich-Noack P, Prehn J H M, Krieglstein J. Stroke. 1996;27:1609–1614. doi: 10.1161/01.str.27.9.1609. [DOI] [PubMed] [Google Scholar]
- 75.Sapolsky R M, Pulsinelli W A. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 76.Abe R, Shimosegawa T, Kikuchi Y, Kimura K, Nagasaki Y, Koizumi M, Toyota T. Pancreas. 1996;12:280–285. doi: 10.1097/00006676-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 77.al Sabbagh A, Nelson P, Akselband Y, Sobel R, Weiner H. Cell Immunol. 1996;171:111–119. doi: 10.1006/cimm.1996.0180. [DOI] [PubMed] [Google Scholar]
- 78.Nussenblatt R B, Whitcup S M, de Smet M D, Caspi R R. Ann NY Acad Sci. 1996;778:325–337. doi: 10.1111/j.1749-6632.1996.tb21140.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z-Y, Qiao J, Link H. J Neuroimmunol. 1993;44:209–214. doi: 10.1016/0165-5728(93)90045-z. [DOI] [PubMed] [Google Scholar]
- 80.Ma C G, Zhang G X, Xiao B G, Wang Z Y, Link J, Olsson T, Link H. Ann NY Acad Sci. 1996;778:273–287. doi: 10.1111/j.1749-6632.1996.tb21135.x. [DOI] [PubMed] [Google Scholar]
- 81.Bergerot I, Fabien N, Mayer A, Thivolet C. Ann NY Acad Sci. 1996;778:362–367. doi: 10.1111/j.1749-6632.1996.tb21144.x. [DOI] [PubMed] [Google Scholar]
- 82.Neurath M, Fuss I, Kelsall B, Presky D, Waegell W, Strober W. J Exp Med. 1996;183:2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peterson K E, Braley-Mullen H. Cell Immunol. 1995;166:123–130. doi: 10.1006/cimm.1995.0014. [DOI] [PubMed] [Google Scholar]
- 84.Hohal M J, Khoury S J, Cook S L, Orav E J, Hafler D A, Weiner H L. Ann NY Acad Sci. 1996;778:243–250. doi: 10.1111/j.1749-6632.1996.tb21132.x. [DOI] [PubMed] [Google Scholar]
- 85.Bitar D M, Whitacre C C. Cell Immunol. 1988;112:364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- 86.McMenamin C, McKersey M, Kuhnlein P, Hunig T, Holt P. J Immunol. 1995;154:4390–4394. [PubMed] [Google Scholar]
- 87.Hoyne G, Thomas W. Immunology. 1995;84:304–309. [PMC free article] [PubMed] [Google Scholar]
- 88.Blanas E, Carbone F R, Allison J, Miller J F A P, Heath W R. Science. 1996;274:1707–1709. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- 89.Genain C P, Abel K, Belamr N, Villinger F, Rosenberg D P, Linington C, Raine C S, Hauser S L. Science. 1996;274:2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]