Abstract
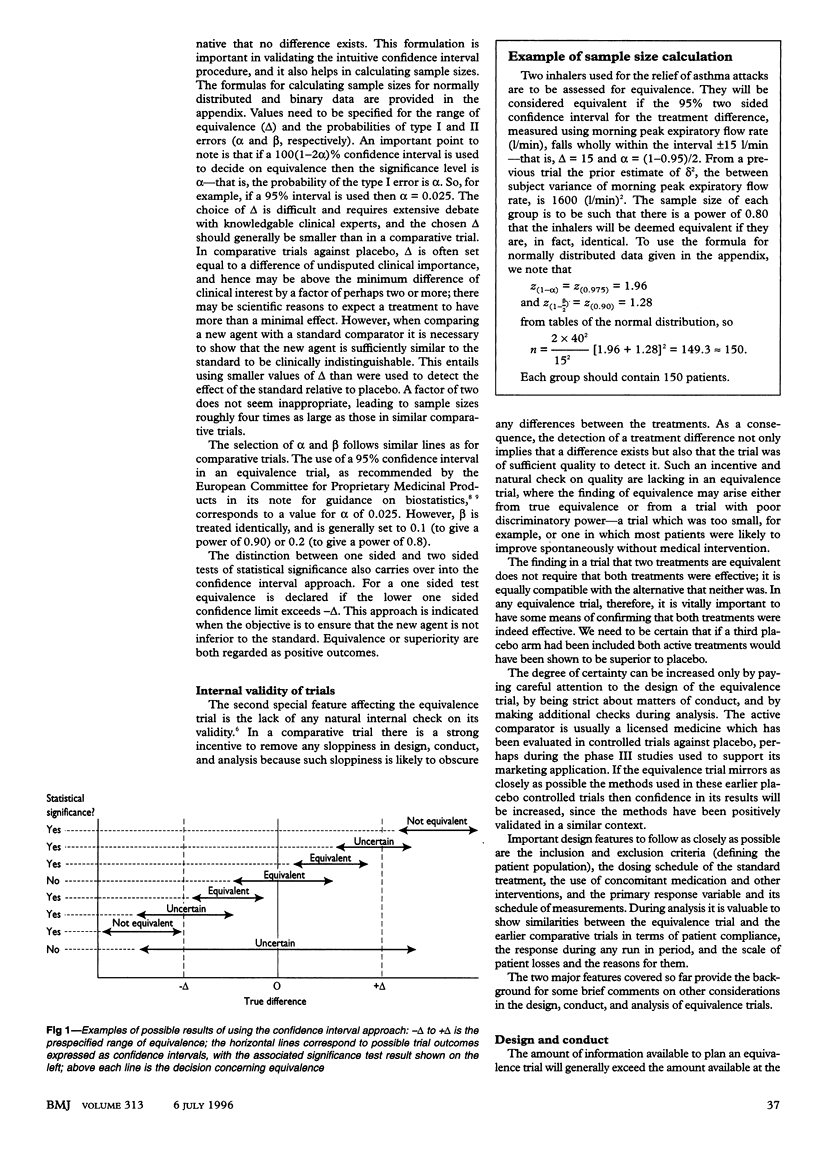
The aim of an equivalence trial is to show the therapeutic equivalence of two treatments, usually a new drug under development and an existing drug for the same disease used as a standard active comparator. Unfortunately the principles that govern the design, conduct, and analysis of equivalence trials are not as well understood as they should be. Consequently such trials often include too few patients or have intrinsic design biases which tend towards the conclusion of no difference. In addition the application of hypothesis testing in analysing and interpreting data from such trials sometimes compounds the drawing of inappropriate conclusions, and the inclusion and exclusion of patients from analysis may be poorly managed. The design of equivalence trials should mirror that of earlier successful trials of the active comparator as closely as possible. Patient losses and other deviations from the protocol should be minimised; analysis strategies to deal with unavoidable problems should not centre on an "intention to treat" analysis but should seek to show the similarity of results from a range of approaches. Analysis should be based on confidence intervals, and this also carries implications for the estimation of the required numbers of patients at the design stage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman D. G., Bland J. M. Absence of evidence is not evidence of absence. BMJ. 1995 Aug 19;311(7003):485–485. doi: 10.1136/bmj.311.7003.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Crombie H. Health and economic policy. BMJ. 1995 Jul 1;311(6996):1–2. doi: 10.1136/bmj.311.6996.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry D., Hill S. Comparing treatments. BMJ. 1995 May 20;310(6990):1279–1279. doi: 10.1136/bmj.310.6990.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Jones D. R., Röhmel J. Biostatistical methodology in clinical trials--a European guideline. Stat Med. 1995 Aug 15;14(15):1655–1657. doi: 10.1002/sim.4780141506. [DOI] [PubMed] [Google Scholar]
- Makuch R., Simon R. Sample size requirements for evaluating a conservative therapy. Cancer Treat Rep. 1978 Jul;62(7):1037–1040. [PubMed] [Google Scholar]
- Rothman K. J., Michels K. B. The continuing unethical use of placebo controls. N Engl J Med. 1994 Aug 11;331(6):394–398. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]
- Taubes G. Use of placebo controls in clinical trials disputed. Science. 1995 Jan 6;267(5194):25–26. doi: 10.1126/science.7809605. [DOI] [PubMed] [Google Scholar]


