Abstract
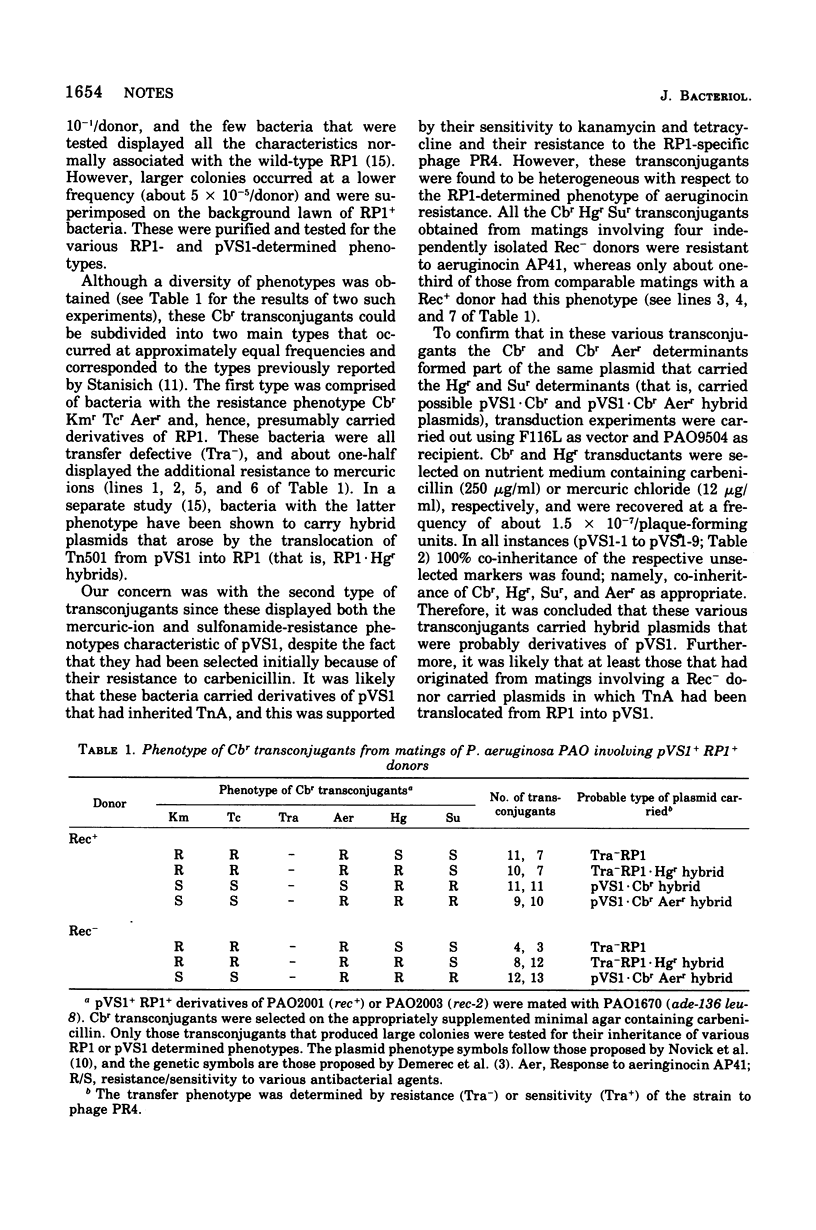
A procedure is described for the isolation, in Pseudomonas aeruginosa PAO, of bacteria carrying derivatives of pVS1 that inherited the carbenicillin-resistance determinant from RP1 either alone or together with that for aeruginocin resistance. Such bacteria occur among the transconjugant progeny from both recombination-proficient or -deficient pVS1+ RP1+ donors, suggesting that the formation of these plasmids is due to the translocation of TnA from RP1 into pVS1. It is possible, therefore, that the aeruginocin-resistance determinant is part of TnA or is closely linked to it. Unexpectedly, none of these plasmids showed the 3 x 10(6)- to 4 x 10(6)-dalton increase in size predicted for TnA+ derivatives of PVS1. It is suggested that an interaction between TnA and the Tn501 translocation unit in pVS1 could account for this result.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Krishnapillai V. Isolation and properties of recombination-deficient mutants of Pseudomonas aeruginosa. Mutat Res. 1974 Apr;23(1):15–23. doi: 10.1016/0027-5107(74)90155-9. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Rossiter H., Burgess D., Dodge J. Aeruginocin tolerant mutants of Pseudomonas aeruginosa. Genet Res. 1973 Dec;22(3):239–253. doi: 10.1017/s0016672300013069. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V. A novel transducing phage. Its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1972;114(2):134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a nonconjugative plasmid in Pseudomonas aeruginosa. J Bacteriol. 1977 Mar;129(3):1227–1233. doi: 10.1128/jb.129.3.1227-1233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M. The properties of hybrids formed between the P-group plasmid RP1 and various plasmids from Pseudomonas aeruginosa. Mol Gen Genet. 1976 Dec 8;149(2):217–223. doi: 10.1007/BF00332892. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A., Holloway B. W. A mutant sex factor of Pseudomonas aeruginosa. Genet Res. 1972 Feb;19(1):91–108. doi: 10.1017/s0016672300014294. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A. Interaction between an R factor and an element conferring resistance to mercuric ions in Pseudomonas aeruginosa. Mol Gen Genet. 1974 Feb 6;128(3):201–212. doi: 10.1007/BF00267109. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A., Ortiz J. M. Similarities between plasmids of the P-incompatibility group derived from different bacterial genera. J Gen Microbiol. 1976 Jun;94(2):281–289. doi: 10.1099/00221287-94-2-281. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A. The properties and host range of male-specific bacteriophages of Pseudomonas aeruginosa. J Gen Microbiol. 1974 Oct;84(2):332–342. doi: 10.1099/00221287-84-2-332. [DOI] [PubMed] [Google Scholar]



