Abstract
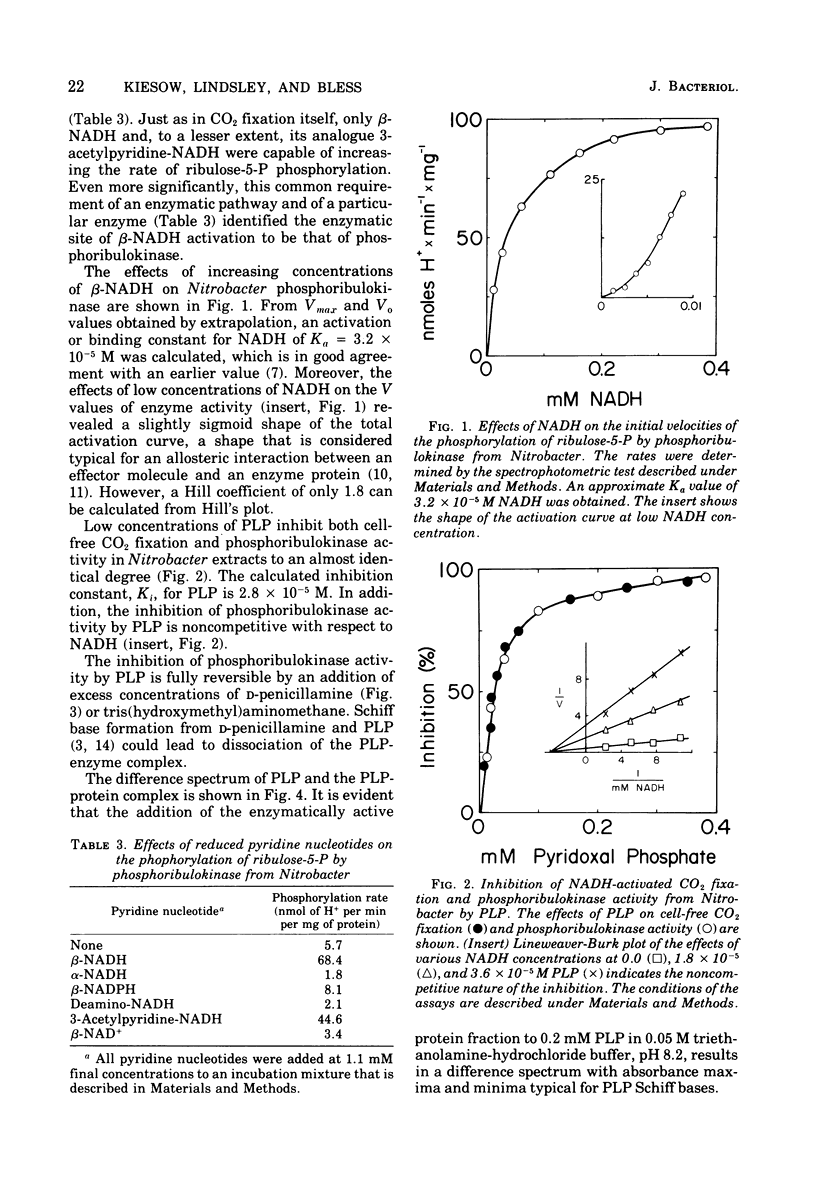
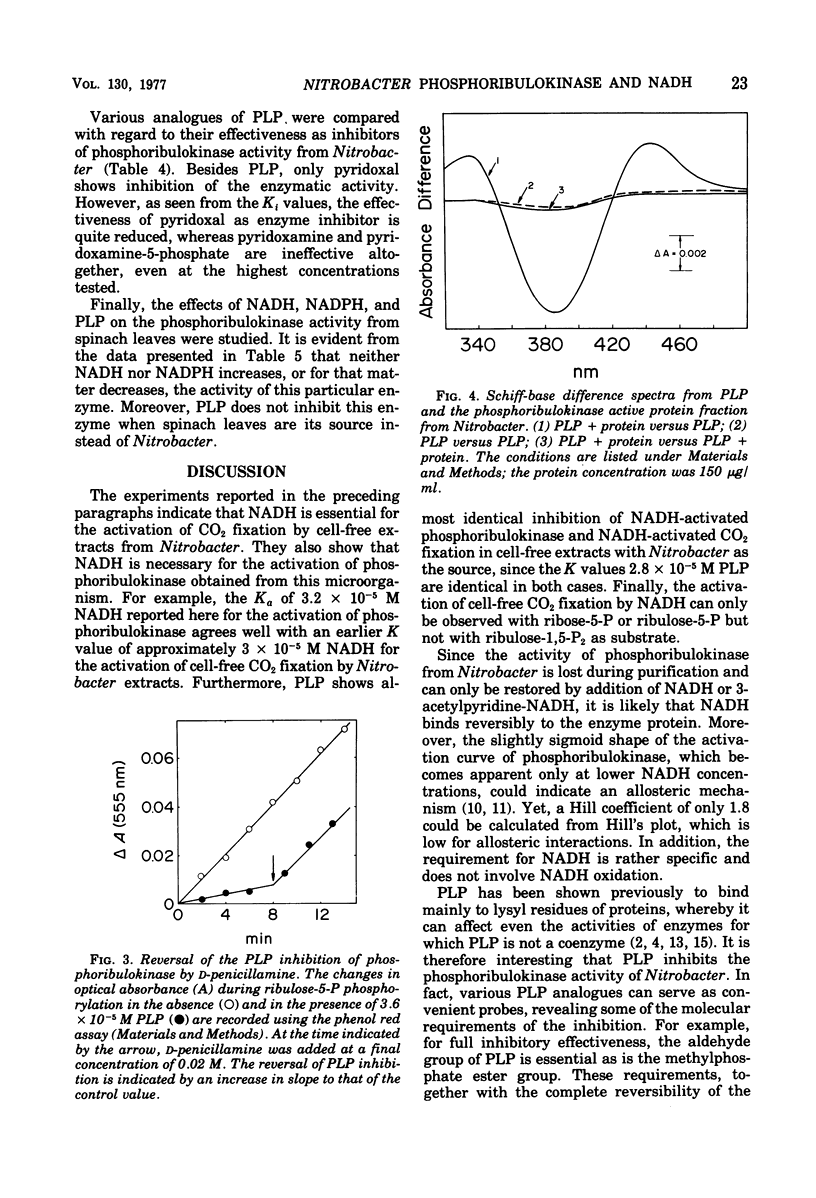
CO2 fixation by particle-free extracts from Nitrobacter winogradskyi increased by addition of reduced nicotinamide adenine dinucleotide (NADH). Ribulose-1,5-diphosphate, however, increased CO2 fixation, even in the absence of NADH. Phosphoribulokinase (EC 2.7.1.19) was the enzyme of Nitrobacter extracts that was activated specifically by NADH. Pyridoxal-5-phosphate inhibited both CO2 fixation and NADH-activated phosphoribulokinase from Nitrobacter. However, it did not affect phosphoribulokinase from spinach leaves. Since the spinach enzyme had also no requirement for reduced pyridine nucleotides, it appears that pyridoxal phosphate interferes only with the binding of NADH and not with the binding of ribulose-5-phosphate and adenosine-5'-triphosphate. The regulation of phosphoribulokinase activity by NADH provided Nitrobacter with an energy-dependent control mechanism of CO2 assimilation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEEM M. I., LEES H. ADENOSINE TRIPHOSPHATE-DEPENDENT REDUCTION OF NICOTINAMIDE ADENINE DINUCLEOTIDE BY FERRO-CYTOCHROME C IN CHEMOAUTOTROPHIC BACTERIA. Nature. 1963 Nov 23;200:759–761. doi: 10.1038/200759a0. [DOI] [PubMed] [Google Scholar]
- Colombo G., Kemp R. G. Specific modification of an effector binding site of phosphofructokinase by pyridoxal phasphate. Biochemistry. 1976 Apr 20;15(8):1774–1780. doi: 10.1021/bi00653a028. [DOI] [PubMed] [Google Scholar]
- HUGHES R. C., JENKINS W. T., FISCHER E. H. The site of binding of pyridoxal-5'-phosphate to heart glutamic-aspartic transaminase. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1615–1618. doi: 10.1073/pnas.48.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenbank G., Körber F., Siegmund P. Uber die Reaktioen von D- und L-Penicillamin mit Alanintransaminase und mit einigen Aldiminen des Pyridoxalphosphats. Hoppe Seylers Z Physiol Chem. 1968 Mar;349(3):310–316. [PubMed] [Google Scholar]
- KIESOW L. ON THE ASSIMILATION OF ENERGY FROM INORGANIC SOURCES IN AUTOTROPHIC FORMS OF LIFE. Proc Natl Acad Sci U S A. 1964 Oct;52:980–988. doi: 10.1073/pnas.52.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesow L. A., Shelton J. B., Bless J. W. CO 2 partial pressure and enthalpy and thermodynamic efficiency of the oxidation of nitrite by Nitrobacter. Arch Biochem Biophys. 1972 Aug;151(2):414–419. doi: 10.1016/0003-9861(72)90516-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Rindt K. P., Ohmann E. NADH and AMP as allosteric effectors of ribulose-5-phosphate kinase in Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1969 Aug 7;36(3):357–364. doi: 10.1016/0006-291x(69)90572-5. [DOI] [PubMed] [Google Scholar]
- Shapiro S., Enser M., Pugh E., Horecker B. L. The effect of pyridoxal phosphate on rabbit muscle aldolase. Arch Biochem Biophys. 1968 Nov;128(2):554–562. doi: 10.1016/0003-9861(68)90062-3. [DOI] [PubMed] [Google Scholar]
- Siegmund P., Hasenbank G., Körber F. Uber die Wirkung der Penicillamine-Antipoden auf Aspartat-transaminase und die Reaktion ihrer Pyridoxal-phosphat-Thiazolidine (3-hydroxy-2-methyl-5-phosphono-oxymethyl-4-(5,5-dimethyl-4-carboxy-thiazolidinyl-(2))-pyridin) mit Apo-aspartattransaminase. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):1062–1070. [PubMed] [Google Scholar]
- Uyeda K. Reaction of phosphofructokinase with maleic anhydride, succinic anhydride, and pyridoxal 5'-phosphate. Biochemistry. 1969 Jun;8(6):2366–2373. doi: 10.1021/bi00834a017. [DOI] [PubMed] [Google Scholar]



