Abstract
Cells with impaired transporter associated with antigen processing (TAP) function express low levels of cell surface major histocompatibility complex (MHC) class I molecules, and are generally resistant to lysis by MHC class I restricted cytotoxic T lymphocytes (CTLs). Here we report the generation of MHC class I restricted CD8+ CTLs that surprisingly require target cell TAP deficiency for efficient recognition. C57BL/6 (B6) mice immunized with syngenic B7–1 (CD80) expressing TAP-deficient cells generated a potent CTL response against both TAP-deficient RMA-S tumor cells and TAP-deficient Con A blasts, whereas the corresponding TAP-expressing target cells were considerably less susceptible or resistant to lysis. The CTL epitopes recognized were expressed also by the human TAP-deficient cell line T2, transfected with appropriate MHC class I molecules. B6 mice immunized with B7–1-transfected TAP-deficient RMA-S cells were protected from outgrowth of a subsequent RMA-S tumor challenge. These findings are discussed in relation to the biochemical nature of MHC class I dependent CTL epitopes associated with impaired TAP function, as well as implications for immunotherapy and autoimmunity.
CD8+ cytotoxic T lymphocytes (CTLs) recognize antigen in the form of short peptides presented by major histocompatibility complex (MHC) class I molecules (1, 2). MHC class I presented peptides are usually 8–11 amino acids in length (3). A majority of these peptides are generated in the cytosol by proteolytic degradation of endogenous proteins (4). The peptides are translocated into the lumen of the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP) (5). In the absence of a functional TAP1/2-heterodimer, most MHC class I molecules are retained in the ER, and only a small fraction are transported to the cell surface (6–9). These MHC class I molecules, often referred to as “empty” or “peptide receptive,” are unstable at physiological temperature but can be stabilized by culture at low temperature or by the addition of exogenous MHC class I binding peptides (10–12).
TAP is considered crucial for MHC class I restricted CTL responses because TAP-deficient cells are inefficiently recognized by conventional MHC class I restricted CTLs specific for viral, minor histocompatibility, or tumor antigens (10, 13, 14). In contrast, TAP-deficient cells can be recognized by some allo-MHC class I specific CTLs (13, 15, 16). It is unclear whether such allo-specific CTLs recognize MHC class I molecules per se or MHC class I molecules loaded with TAP-independent peptides. The latter may include peptide species derived from signal sequences (17, 18) or peptides imported to the ER by other TAP-independent mechanisms.
In this study, we have addressed whether TAP-deficient cells can elicit a CTL response in the syngenic host. This is an important question because putative TAP-independent CTL epitopes could serve as rejection antigens in tumors that have lost TAP expression (19–21) and in certain virus infections where TAP function is inhibited by viral proteins, e.g., in Herpes simplex virus infected cells (22, 23). Furthermore, immunization strategies based on TAP-deficient cells as carriers of exogenously added MHC class I presented peptides (14, 24–26) require knowledge about the immune response to TAP-deficient cells as such. This study demonstrates the existence of novel MHC class I restricted CTL epitopes that are recognized on cells with impaired TAP function.
MATERIALS AND METHODS
Mice.
All mice used were bred and maintained at the Microbiology and Tumor Biology Center (Karolinska Institute, Stockholm). The generation and characterization of TAP1−/−, β2-microglobulin (β2m)−/−, and TAP1/β2m−/− mice has been described (27–29). The TAP1−/− and β2m−/− mice used in this study have been backcrossed to C57BL/6 (B6) mice at least six times. Animal care was in accordance with institutional guidelines.
Cell Lines.
RMA-S is a TAP2-deficient tumor cell line, derived from the Rauscher leukemia virus-induced mouse T cell lymphoma RBL-5 of B6 origin (30). RMA-S II 5.9 cells, here referred to as RMA-S.TAP2 cells, were derived by transfection of RMA-S with the murine TAP2 gene (31). T2Kb is a H-2Kb transfected subline of the TAP1/2-deficient mutant human cell line T2 (32). P815 is a mastocytoma of H-2d haplotype, induced by benzpyrene in the DBA/2 mouse strain. Cell lines were grown in RPMI 1640 medium supplemented with penicillin/streptomycin and 5% fetal calf serum (FCS) or serum-free AIM-V medium (Life Technologies, Gaithersburg, MD) in 37°C and 5% CO2/95% air.
Transfection of RMA-S with B7–1 (CD80).
RMA-S cells (2 × 106) were incubated with 10 μl Lipofectamine (Life Technologies) and 1 μg of the murine B7–1 gene cloned in a pBR322 plasmid (a gift from Bristol–Myers Squibb). Transfected cells were selected on Geneticin (Life Technologies) at a concentration of 1 mg/ml. The 1% most positive fraction of the B7–1 expressing RMA-S cells were sorted on a FACS Vantage cell sorter (Becton Dickinson) and designated RMA-S.B7–1.
Antibodies.
B7–1 expression was assessed either with the CTLA-4Ig fusion protein (33), a kind gift from P. Lane (Basel Institute for Immunology), a fluorescein isothiocyanate (FITC)-conjugated anti-human IgG antibody (Dako), or with biotinylated anti-B7–1 monoclonal antibody 16–10A1 (PharMingen) and Neutralite avidin–FITC (Southern Biotechnology Associates).
Generation of Con A Activated T Cell Blasts.
Spleen cells were incubated for 48 hr at 2 × 106 cells/ml in α-MEM medium supplemented with penicillin/streptomycin, 10% FCS, 10 mM Hepes, 3 × 10−5 M 2-mercaptoethanol and 3 μg/ml Con A (Sigma). Before use as targets in a standard 4 hr 51Cr release cytotoxicity assay, dead cells were removed by centrifugation on a Lymphoprep gradient (Nycomed, Oslo).
In Vivo Immunization.
B6 mice were immunized with three weekly s.c. inoculations of 10 × 106 irradiated [100 Gray (Gy)] tumor cells or 50 × 106 irradiated (20 Gy) spleen cells. Tumor cells used were serially passaged as ascites cell lines in 4 Gy irradiated (syngenic or H-2 compatible) mice.
In Vitro Stimulation.
Single cell suspensions of spleens from immunized or nonimmunized mice were prepared. Effector cells (20 × 106) were incubated with 2 × 106 (100 Gy) irradiated tumor cells or 20 × 106 (20 Gy) irradiated spleen cells. Cultures were kept in 10 ml of RPMI medium 1640 supplemented with penicillin/streptomycin, 10% FCS, 3 × 10−5 M 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 2 mM l-glutamine in 37°C and 5% CO2/95% air for 5 days.
In Vitro Cytotoxicity Assay.
In vitro cytotoxicity was measured in a standard 51Cr-release assay. Briefly, target cells were labeled with 51Cr and resuspended in cell culture medium. Target cells (5 × 103) were added to each well followed by the addition of effector cells. The cells were incubated for 4 hr at 37°C and supernatants were harvested. Radioactivity was measured in a Pharmacia LKB γ-counter, and specific lysis was calculated [(CPM released with effector cells − CPM released without effector cells)/(CPM released by detergent − CPM released without effector cells)] × 100. For experiments performed in serum-free AIM-V media, effector cells were washed twice in AIM-V media before addition to target cells. Ascites passaged tumor target cells were grown for at least 2 weeks in AIM-V media. For cold target inhibition experiments, effector cells and nonlabeled (cold) target cells were mixed and 51Cr-labeled (hot) target cells were subsequently added to this mixture.
Complement-Mediated Depletion of Effector Populations.
Effector cells (20 × 106) were incubated in 1 ml of anti-CD8 antibody (a mixture of 169.4 and 156.7.7, generously provided by H. Waldmann, Cambridge University) or in 1 ml of anti-CD4 antibody (YTS191, from the European Collection of Cell Cultures, Salisbury, U.K.) for 60 min at 4°C. Antibodies were at a concentration of 200 μg/ml. The cells were washed once and incubated with rabbit complement (Pel-Freez Biologicals) diluted 1:8 for 75 min at 37°C. Before use as effectors, the cells were washed twice in PBS and diluted in cell culture media.
In Vivo Tumor Growth.
B6 mice were immunized as described. One week after the last immunization, the mice were given 106 live tumor cells s.c. and tumor growth was followed by weekly palpations. For each mouse the experiment was terminated when the tumor reached a diameter of 20 mm.
RESULTS
RMA-S Cells Transfected with B7–1 (CD80) Elicit CTLs That Recognize RMA-S Cells.
TAP2-deficient RMA-S tumor cells were inefficient in eliciting cytotoxic responses in B6 mice. In contrast, immunization of B6 mice with RMA-S cells transfected with B7–1 (RMA-S.B7–1) resulted in a strong and reproducible secondary cytotoxic response in vitro against nontransfected RMA-S targets (Fig. 1 A and B). The cytotoxicity could be abrogated by pretreatment of effectors with anti-CD8 antibodies and complement (Fig. 1C). The response was not directed against FCS components, because RMA-S cells cultured and tested for cytotoxicity in serum-free medium were killed as efficiently as cells grown in FCS containing media (data not shown). Furthermore, the priming in vivo was always performed with RMA-S.B7–1 or RMA-S cells from cell lines passaged in vivo for several generations. We thus conclude that it is possible to generate a CD8+ CTL response against syngenic TAP-deficient cells.
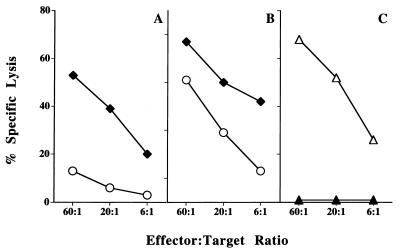
Figure 1.
Immunization of B6 mice with RMA-S.B7–1 cells elicits CD8+ CTLs that recognize nontransfected RMA-S cells. (A and B) B6 mice were immunized in vivo and splenocytes were restimulated in vitro with RMA-S cells (○) or RMA-S cells transfected with B7–1 (⧫) and tested for cytotoxicity against RMA-S target cells. Two experiments are shown; A is representative of 4/6 experiments, B is representative of the remaining two experiments. (C) CTLs generated as above were depleted with anti-CD8 antibodies and complement (▴) or anti-CD4 antibodies and complement (▵) and tested for cytotoxicity against RMA-S target cells. One representative experiment of three is shown.
Efficient CTL Recognition of TAP-Deficient Cells Requires the Presence of MHC Class I Molecules and the Absence of TAP in the Target Cell.
A TAP2 transfectant of RMA-S (RMA-S.TAP2) was considerably less susceptible to lysis by RMA-S.B7–1 elicited CTLs (Fig. 2A). This indicated that the CTL epitopes recognized were expressed preferentially in cells deficient in TAP expression. In line with this, Con A blasts from TAP1−/− mice were highly sensitive to lysis by these CTLs, whereas Con A blasts from B6 mice were resistant to lysis. Con A blasts from TAP1/β2m−/− (double mutant) mice were resistant to lysis, suggesting an MHC class I dependence in the CTL recognition (Fig. 2B). Furthermore, the human TAP-deficient cell line T2 transfected with H-2Kb was sensitive to lysis by RMA-S.B7–1 elicited CTLs, whereas nontransfected T2 cells were resistant (Fig. 2C). Taken together, these results suggest that the response elicited by RMA-S.B7–1 is MHC class I specific or restricted and directed against epitopes expressed preferentially by TAP-deficient cells. At least some of the epitopes could be recognized on both nontransformed and transformed lymphoid cells, and on cells of both human and murine origin. We will refer to these epitopes as epitopes associated with impaired TAP function. The low levels of killing of RMA-S.TAP2 were investigated further by cold target competition studies. Cold RMA-S.TAP2 and P815 cells did not compete for RMA-S killing in these experiments. However, cold RMA-S.TAP2 gave a dose-dependent inhibition of RMA-S.TAP2 killing (data not shown). Whereas these data do not formally exclude expression of epitopes associated with impaired TAP function on TAP-expressing tumor cells, they suggest that the CTL epitopes recognized were expressed preferentially in tumor cells deficient in TAP expression. Furthermore, additional cold target competition studies showed that Con A blasts from TAP1−/− mice did compete for killing of RMA-S, whereas Con A blasts from B6 mice did not (data not shown).
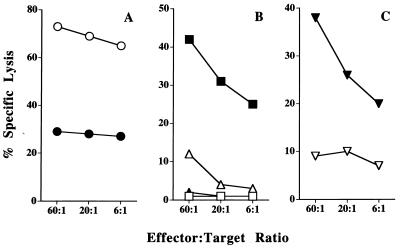
Figure 2.
Efficient recognition of epitopes by RMA-S.B7–1 elicited CTLs requires the absence of TAP and the presence of MHC class I molecules in the target cell. B6 mice were immunized in vivo and splenocytes were restimulated in vitro with RMA-S.B7–1 cells and tested for cytotoxicity against (A) RMA-S (○) and RMA-S.TAP-2 (•) cells; (B) Con A blasts from B6 (□), TAP1−/− (▪), β2m−/− (▵), and TAP1/β2m−/− mice (▴); and (C) T2 (▿) and T2Kb (▾) cells. One representative experiment of three is shown.
TAP1−/− Splenocytes Elicit CTLs That Recognize TAP1−/− Con A Blasts and RMA-S Tumor Cells.
CTLs elicited by RMA-S.B7–1 recognized cells from TAP1−/− mice. Accordingly, immunization of B6 mice with splenocytes from TAP1−/− mice yielded CTLs that efficiently lysed TAP1−/− Con A blasts and RMA-S tumor cells, whereas B6 Con A blasts were resistant and RMA-S.TAP2 cells were killed at markedly reduced levels (Fig. 3; data not shown).
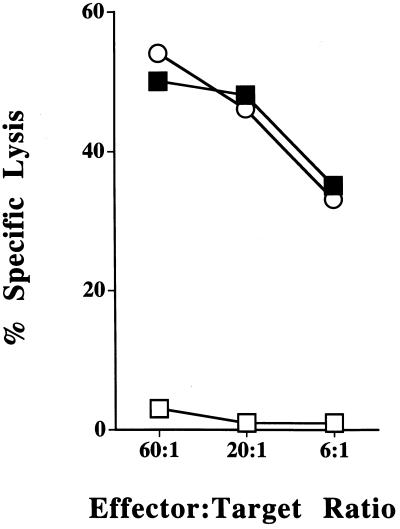
Figure 3.
Immunization with TAP1−/− splenocytes elicits CTLs that recognize TAP1−/− Con A blasts and RMA-S tumor cells. B6 mice were immunized in vivo and splenocytes were restimulated in vitro with splenocytes from TAP1−/− mice and tested for cytotoxicity against RMA-S (○), B6 Con A blasts (□), and TAP1−/− Con A blasts (▪). One representative experiment of four is shown.
Generation of Primary CTLs Specific for TAP-Deficient Cells.
Stimulation in vitro, without prior in vivo immunization, of B6 spleen cells with RMA-S.B7–1 or TAP1−/− spleen cells reproducibly resulted in cytotoxic responses against RMA-S and TAP1−/− Con A blasts targets, whereas RMA-S.TAP2 and B6 Con A blasts were considerably less sensitive (Table 1). The killing of RMA-S and TAP1−/− Con A blast targets was abrogated by pretreatment of effectors with anti-CD8 antibody and complement (data not shown), confirming that the lysis was mediated by CD8+ CTLs.
Immunization of B6 Mice with RMA-S.B7–1 Protects Against RMA-S Tumor Growth.
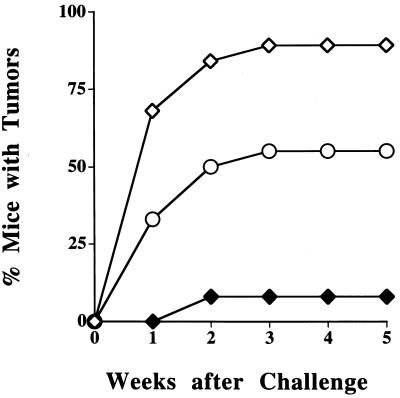
To address whether it was possible to elicit a protective immune response against a TAP-deficient tumor, we immunized B6 mice with RMA-S cells, RMA-S.B7–1 cells or PBS. After three weekly immunizations, mice were challenged with 106 live RMA-S tumor cells s.c., a dose previously found to overcome the natural killer mediated rejection of RMA-S (30). The tumor cells used for immunization and challenge were always from in vivo passaged lines that had not been exposed to FCS. Eighty-nine percent of the mice (17/19) immunized with PBS developed progressively growing tumors within 3 weeks of the challenge. In contrast, only 8% (1/13) of the mice immunized with RMA-S.B7–1 developed tumors. Mice immunized with RMA-S were partially protected; 55% of the mice (10/18) developed progressively growing tumors (Fig. 4). The RMA-S.B7–1 mediated protection from tumor growth was also seen in mice depleted of natural killer cells (data not shown).
Figure 4.
Immunization of B6 mice with RMA-S.B7–1 cells protects these mice from the outgrowth of RMA-S tumor cells. B6 mice were given 106 live RMA-S tumor cells after immunization with PBS (⋄), RMA-S (○), or RMA-S.B7–1 (⧫) cells. This figure represents accumulated data from four separate experiments (three for RMA-S.B7–1) with 4–6 mice per group in each experiment.
DISCUSSION
This study demonstrates the generation of CD8+ T cells specific for syngenic TAP-deficient cells. B6 mice immunized with B7–1 transfected TAP-deficient RMA-S tumor cells or spleen cells from TAP1−/− mice generated a potent CTL response against both RMA-S tumor cells and TAP1−/− Con A blast targets. In contrast, TAP-expressing RMA-S.TAP2 tumor cells were considerably less sensitive and B6 Con A blasts were resistant to lysis by these CTLs. RMA-S.B7–1 immunized mice were protected from outgrowth of RMA-S tumor transplants, indicating that the epitopes recognized can function as tumor rejection antigens in vivo. In vitro killing as well as in vivo rejection was observed with cells grown in the absence of FCS.
What is the nature of the epitopes associated with impaired TAP function? The results from experiments with Con A blasts from TAP1−/− and TAP1/β2m−/− mice, as well as H-2Kb transfected and control T2 cells, show that at least a part of the response is directed against epitopes that depend on the expression of MHC class I molecules. The MHC class I molecules on TAP-deficient cells can either be loaded with a set of peptides that are not presented by TAP-expressing cells, or alternatively, the MHC class I molecules on TAP-deficient cells display an altered conformation that in itself creates novel epitopes. With respect to the first possibility, it is clear that peptides can be loaded in MHC class I molecules in a TAP-independent manner. Signal sequence derived peptides have been isolated from MHC class I molecules of TAP-deficient T2 cells (17, 18), and a signal sequence preceding a TAP-dependent epitope resulted in presentation of the epitope in TAP-deficient cells (19, 34). Furthermore, TAP-deficient RMA-S cells are “leaky” and present certain viral and minor histocompatibility antigens on MHC class I molecules, albeit at a low level (31, 35, 36). In addition, certain viral epitopes may be presented not only in the absence of TAP but through a pathway that does not even depend on de novo export of MHC class I molecules from the ER through the secretory pathway (37). However, the epitopes recognized in this study may not simply be explained by “leakiness” in TAP-deficient cells or alternative pathways, since their expression seem to be inhibited by a functional TAP complex. One possible explanation could be an uneven competition for MHC class I molecules between two sets of peptides in the ER, where one set would be bound sufficiently only in the absence of TAP-imported peptides. Extensive efforts to isolate peptides that may represent the epitopes associated with impaired TAP function, by elution from whole cell lysates or purified MHC class I molecules of RMA-S cells, have not been fruitful, although we have been successful using an identical approach for the isolation of TAP-dependent tumor and minor histocompatibility epitopes from RMA, RMA-S.TAP2, B6, and BALB.B spleen cells (refs. 14 and 38; data not shown). It may be that the tentative peptides are difficult to isolate due to low levels of expression or different chemical properties than those of the previously defined TAP-dependent peptides. Alternatively, the epitopes may not be of peptidic nature. Another possibility is that the epitopes are formed by the MHC class I molecules themselves. The exact structure of MHC class I molecules on TAP-deficient cells is not known. They may express an altered conformation caused by either the absence of a bound peptide or by virtue of binding a peptide with low affinity (11, 39). The observation that H-2Kb transfected T2 cells express the epitopes associated with impaired TAP function can be interpreted within the frame of both models proposed. If the epitopes represents antigens loaded in class I molecules, this observation indicates that some of them must be conserved between mouse and man. Whereas TAP-expressing Con A blasts were resistant for the CTLs described, the TAP-expressing tumor cell RMA-S.TAP2 was killed at low levels. We can therefore not exclude that some TAP-independent epitopes can be expressed on tumor cells with intact TAP function, although cold target competition studies did not support this (data not shown). In this context, it may be noted that some tumor cells show reduced TAP function even though they contain structurally intact TAP genes (21).
B7–1 (CD80) markedly enhanced the anti-RMA-S CTL response. This is compatible with a suggested model where the role of B7–1 is to decrease the threshold for T cell activation (40–42). The TAP-expressing cell lines RMA and RMA-S.TAP-2 elicit a potent CTL response in B6 mice (ref. 14; data not shown), despite the lack of expression of B7–1/B7–2. It could be that TAP affects the expression of other costimulatory molecules or that the TAP-dependent epitopes of RMA have different costimulatory requirements than the epitopes associated with impaired TAP function. However, after immunization with intact cells, the activation of a T cell response may be dependent on crosspriming, i.e., the destruction of cells followed by uptake and presentation of epitopes on professional antigen-presenting cells (43, 44). Crosspriming may not allow the epitopes associated with impaired TAP function to be efficiently presented, because low-affinity binding peptides or “empty” molecules would not be expressed by a professional TAP-expressing APC. The introduction of B7 may turn the TAP-deficient tumor cell into an antigen-presenting cell, thus bypassing the need for crosspriming (45). The exact roles of direct versus crosspriming of CTLs to different epitopes remain to be investigated in detail. The CTL response against RMA-S.B7–1 was surprisingly potent. One explanation could be related to the high levels of B7–1 expression on this cell line. Another explanation could be that TAP-deficient cells may present the epitopes at high ligand density, despite low expression of MHC class I molecules. Within the frame of the peptide model for the epitopes associated with impaired TAP function it may be argued that the number of different peptides in the ER is relatively low in this situation, and one or a few peptide species therefore dominate the MHC class I pool. On the other hand, if the epitopes are formed by the MHC class I molecules themselves, every molecule on the cell surface is a potential ligand. One must also consider the possibility that epitopes are expressed at low absolute ligand density, but that the absence of other MHC–peptide complexes enhances the likelihood for T cell receptor engagement. This would be in line with the observation that peptide loaded TAP-deficient cells are more efficient in priming peptide specific CTLs in vitro than their TAP intact counterparts (24, 26, 46), although the TAP intact cells in some cases have been shown to express more peptide receptive molecules (12). A third explanation for the potent CTL response is that naive B6 mice have high numbers of precursors to these epitopes.
Many human tumors of different origin have lost or down-regulated MHC class I expression (47), which in some cases is attributed to loss of TAP function (19–21). Such MHC class I-deficient tumors are generally presumed to be resistant to CTL-mediated immunosurveillance and immunotherapy. It has previously been suggested that immunization with cells, where MHC class I expression and presentation has been restored, would potentiate the CTL response to the MHC-deficient counterpart (48). However, restoration of the MHC class I antigen presentation pathway may in some situations inhibit a specific CTL response, either by eliminating epitopes specific for the MHC-deficient state or by other mechanisms of immunodominance (38, 49–52). In this study, we have used an alternative strategy that involves the generation of systemic immunity by immunization with a B7–1 transfected tumor retaining the impaired MHC class I expression. Such tumor immunization has previously been described for several MHC class I expressing tumors (53). Vaccination with RMA-S.B7–1 protected from tumor growth of RMA-S cells, whereas vaccination with nontransfected RMA-S cells gave some, but less efficient, protection. It is likely that CTLs directed to epitopes associated with impaired TAP function were responsible for protection from tumor growth in vivo. Such CTLs might be exploited for immunotherapy against TAP-deficient tumor variants, which can escape from conventional MHC class I restricted CTL responses. This study suggests that it may even be possible to induce immunity against TAP-deficient tumors by immunization with syngenic nontransformed cells lacking TAP function. Another implication of medical interest is that self epitopes, exposed only upon TAP inhibition and toward which no tolerance is established, may be targets for pathogenic as well as therapeutical CTLs in virus infections where TAP function is inhibited (22, 23).
In conclusion, the finding that CTLs can be elicited by epitopes associated with impaired TAP function may provide new target structures in tumor, auto-, and viral immunity, and add to our understanding of antigen-presenting mechanisms and selection of immunogenic epitopes for CTL recognition.
Table 1.
Generation of primary CTL specific for TAP-deficient cells
| Effector cells | Effector/target ratio | Target cells
|
||||
|---|---|---|---|---|---|---|
| TAP1−/−* | β2m−/− | B6 | RMA-S | RMA-S.TAP2 | ||
| B6 anti-TAP1−/− | 60:1 | 77† | 22 | 26 | 46 | 20 |
| 20:1 | 58 | 4 | 24 | 25 | 12 | |
| 7:1 | 41 | 2 | 18 | 11 | 8 | |
| B6 anti-RMA-S.B7-1 | 60:1 | 48 | 14 | 1 | 38 | 21 |
| 20:1 | 25 | 10 | 0 | 20 | 16 | |
| 7:1 | 14 | 3 | 0 | 10 | 8 | |
| B6 anti-RMA-S | 60:1 | 0 | 4 | 0 | 7 | 4 |
| 20:1 | ND | ND | ND | ND | ND | |
| 7:1 | ND | ND | ND | ND | ND | |
The table shows one representative experiment out of three. ND, not done.
TAP1−/−, β2m−/−, and B6 target cells were Con A blasts.
Results are expressed as percent specific lysis.
Acknowledgments
We thank M.-L. Solberg and M. Hagelin for excellent technical assistance, Drs. L. Van Kaer, P. Lane, and H. Waldmann for generous gifts of mutant mice and reagents, and Dr. G. Klein for helpful discussions. This work was supported by grants from the Swedish Cancer Society, the Swedish Medical Research Council, the Karolinska Institute, the Swedish Society for Medical Research, the Göran Gustafsson Foundation, and Arbetsmarknadens Försäkringsaktiebolag.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CTLs, cytotoxic T lymphocytes; MHC, major histocompatibility complex; ER, endoplasmic reticulum; TAP, transporter associated with antigen processing; β2m, β2-microglobulin; B6, C57BL/6.
References
- 1.Townsend A R, Rothbard J, Gotch F M, Bahadur G, Wraith D, McMichael A J. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkman P J, Saper M A, Samraoui B, Bennett W S, Strominger J L, Wiley D C. Nature (London) 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 3.Falk K, Rötzschke O, Stevanovic S, Jung G, Rammensee H G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 4.Groettrup M, Soza A, Kuckelkorn U, Kloetzel P-M. Immunol Today. 1996;9:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 5.Heemels M T, Ploegh H. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 6.Powis S J, Townsend A R, Deverson E V, Bastin J, Butcher G W, Howard J C. Nature (London) 1991;354:528–531. doi: 10.1038/354528a0. [DOI] [PubMed] [Google Scholar]
- 7.Spies T, DeMars R. Nature (London) 1991;351:323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- 8.Degen E, Cohen-Doyle M F, Williams D B. J Exp Med. 1992;175:1653–1661. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh W K, Mitchell E K, Yang Y, Peterson P A, Waneck G L, Williams D B. J Exp Med. 1996;184:337–348. doi: 10.1084/jem.184.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend A, Öhlén C, Bastin J, Ljunggren H G, Foster L, Kärre K. Nature (London) 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 11.Ljunggren H G, Stam N J, Öhlén C, Neefjes J J, Höglund P, Heemels M T, Bastin J, Schumacher T N M, Townsend A, Kärre K. Nature (London) 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 12.Day P M, Esquivel F, Lukszo J, Bennink J R, Yewdell J W. Immunity. 1995;2:137–147. doi: 10.1016/s1074-7613(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 13.Öhlén C, Bastin J, Ljunggren H G, Foster L, Wolpert E, Klein G, Townsend A R, Kärre K. J Immunol. 1990;145:52–58. [PubMed] [Google Scholar]
- 14.Franksson L, Petersson M, Kiessling R, Kärre K. Eur J Immunol. 1993;23:2606–2613. doi: 10.1002/eji.1830231034. [DOI] [PubMed] [Google Scholar]
- 15.Aosai F, Öhlén C, Ljunggren H G, Höglund P, Franksson L, Ploegh H, Townsend A, Kärre K, Stauss H J. Eur J Immunol. 1991;21:2767–2774. doi: 10.1002/eji.1830211118. [DOI] [PubMed] [Google Scholar]
- 16.Rötzschke O, Falk K, Faath S, Rammensee H G. J Exp Med. 1991;174:1059–1071. doi: 10.1084/jem.174.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei M L, Cresswell P. Nature (London) 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 18.Henderson R A, Cox A L, Sakaguchi K, Appella E, Shabanowitz J, Hunt D F, Engelhard V H. Proc Natl Acad Sci USA. 1993;90:10275–10279. doi: 10.1073/pnas.90.21.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Restifo N P, Esquivel F, Kawakami Y, Yewdell J W, Mule J J, Rosenberg S A, Bennink J R. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cromme F V, Airey J, Heemels M T, Ploegh H L, Keating P J, Stern P L, Meijer C J, Walboomers J M. J Exp Med. 1994;179:335–340. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seliger B, Maeurer M J, Ferrone S. Immunol Today. 1997;18:292–299. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 22.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Nature (London) 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 23.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. Nature (London) 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 24.Bellone M, Iezzi G, Manfredi A A, Protti M P, Dellabona P, Casorati G, Rugarli C. Eur J Immunol. 1994;24:2691–2698. doi: 10.1002/eji.1830241118. [DOI] [PubMed] [Google Scholar]
- 25.Mandelboim O, Berke G, Fridkin M, Feldman M, Eisenstein M, Eisenbach L. Nature (London) 1994;369:67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- 26.Nair S K, Snyder D, Gilboa E. J Immunol. 1996;156:1772–1780. [PubMed] [Google Scholar]
- 27.Koller B H, Marrack P, Kappler J W, Smithies O. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 28.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 29.Ljunggren H G, Van Kaer L, Sabatine M S, Auchincloss H, Jr, Tonegawa S, Ploegh H L. Int Immunol. 1995;7:975–984. doi: 10.1093/intimm/7.6.975. [DOI] [PubMed] [Google Scholar]
- 30.Kärre K, Ljunggren H G, Piontek G, Kiessling R. Nature (London) 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Glas R, Momburg F, Hämmerling G J, Jondal M, Ljunggren H G. Eur J Immunol. 1993;23:1796–1801. doi: 10.1002/eji.1830230810. [DOI] [PubMed] [Google Scholar]
- 32.Cerundolo V, Alexander J, Anderson K, Lamb C, Cresswell P, McMichael A, Gotch F, Townsend A. Nature (London) 1990;345:449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- 33.Lane P, Gerhard W, Hubele S, Lanzavecchia A, McConnell F. Immunology. 1993;80:56–61. [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. J Exp Med. 1991;174:489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosken N A, Bevan M J. J Exp Med. 1992;175:719–729. doi: 10.1084/jem.175.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esquivel F, Yewdell J, Bennink J. J Exp Med. 1992;175:163–168. doi: 10.1084/jem.175.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Glas R, Liu T, Ljunggren H G, Jondal M. Eur J Immunol. 1993;23:1802–1808. doi: 10.1002/eji.1830230811. [DOI] [PubMed] [Google Scholar]
- 38.Wolpert E, Franksson L, Kärre K. Int Immunol. 1995;7:919–928. doi: 10.1093/intimm/7.6.919. [DOI] [PubMed] [Google Scholar]
- 39.Parham P. Nature (London) 1990;346:793–795. doi: 10.1038/346793a0. [DOI] [PubMed] [Google Scholar]
- 40.Bluestone J A. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 41.Green J M, Noel P J, Sperling A I, Walunas T L, Gray G S, Bluestone J A, Thompson C B. Immunity. 1994;1:501–508. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 42.Johnston J V, Malacko A R, Mizuno M T, McGowan P, Hellström I, Hellström K E, Marquardt H, Chen L. J Exp Med. 1996;183:791–800. doi: 10.1084/jem.183.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bevan M J. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 45.Huang A Y C, Bruce A T, Pardoll D M, Levitsky H I. J Exp Med. 1996;183:769–776. doi: 10.1084/jem.183.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Bruijn M L, Schumacher T N, Nieland J D, Ploegh H L, Kast W M, Melief C J. Eur J Immunol. 1991;21:2963–2970. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- 47.Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern P L. Immunol Today. 1993;14:491–499. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- 48.Porgador A, Feldman M, Eisenbach L. Nat Immun. 1994;13:113–130. [PubMed] [Google Scholar]
- 49.Korngold R, Wettstein P J. J Immunol. 1990;145:4079–4088. [PubMed] [Google Scholar]
- 50.Glas R, Franksson L, Öhlén C, Höglund P, Koller B, Ljunggren H G, Kärre K. Proc Natl Acad Sci USA. 1992;89:11381–11385. doi: 10.1073/pnas.89.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sercarz E E, Lehmann P V, Ametani A, Benichou G, Miller A, Moudgil K. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 52.Hill A B, Mullbacher A, Blanden R V. Immunol Rev. 1993;133:75–91. doi: 10.1111/j.1600-065x.1993.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 53.Hellström K E, Hellström I, Chen L. Immunol Rev. 1995;145:123–145. doi: 10.1111/j.1600-065x.1995.tb00079.x. [DOI] [PubMed] [Google Scholar]