Abstract
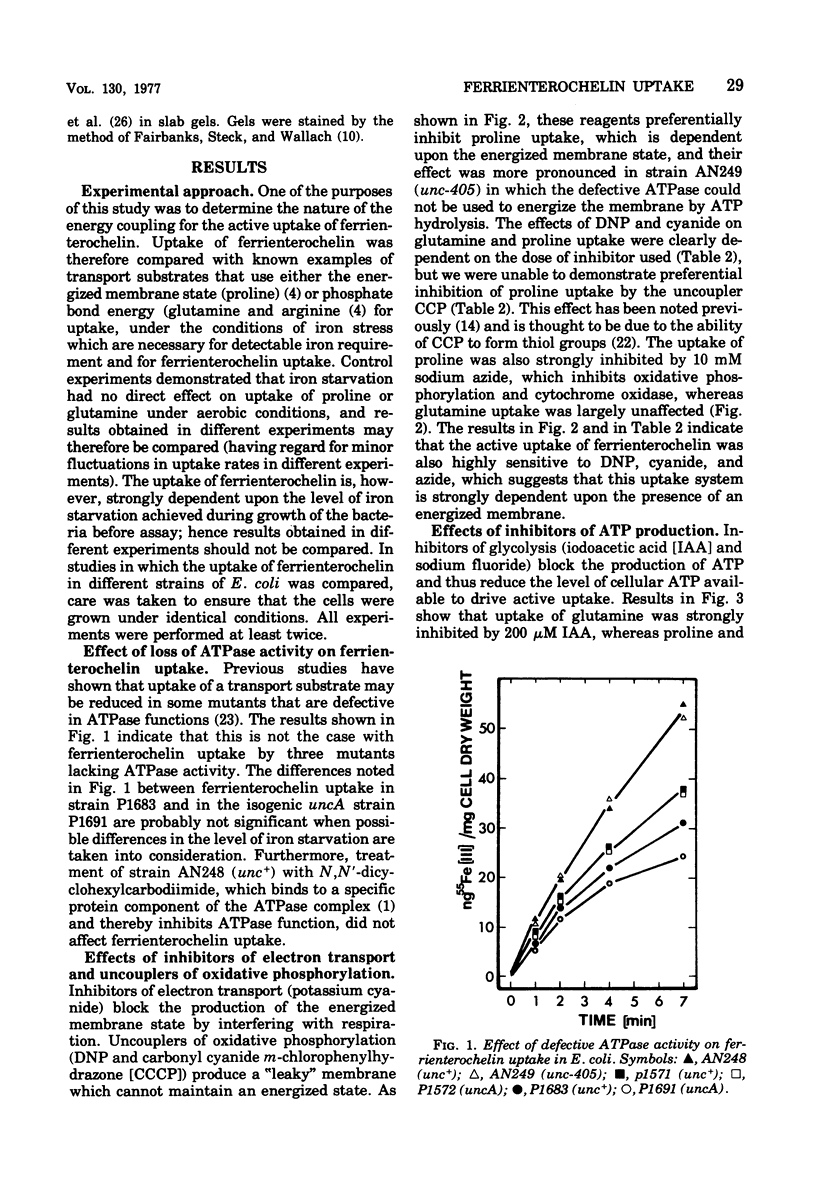
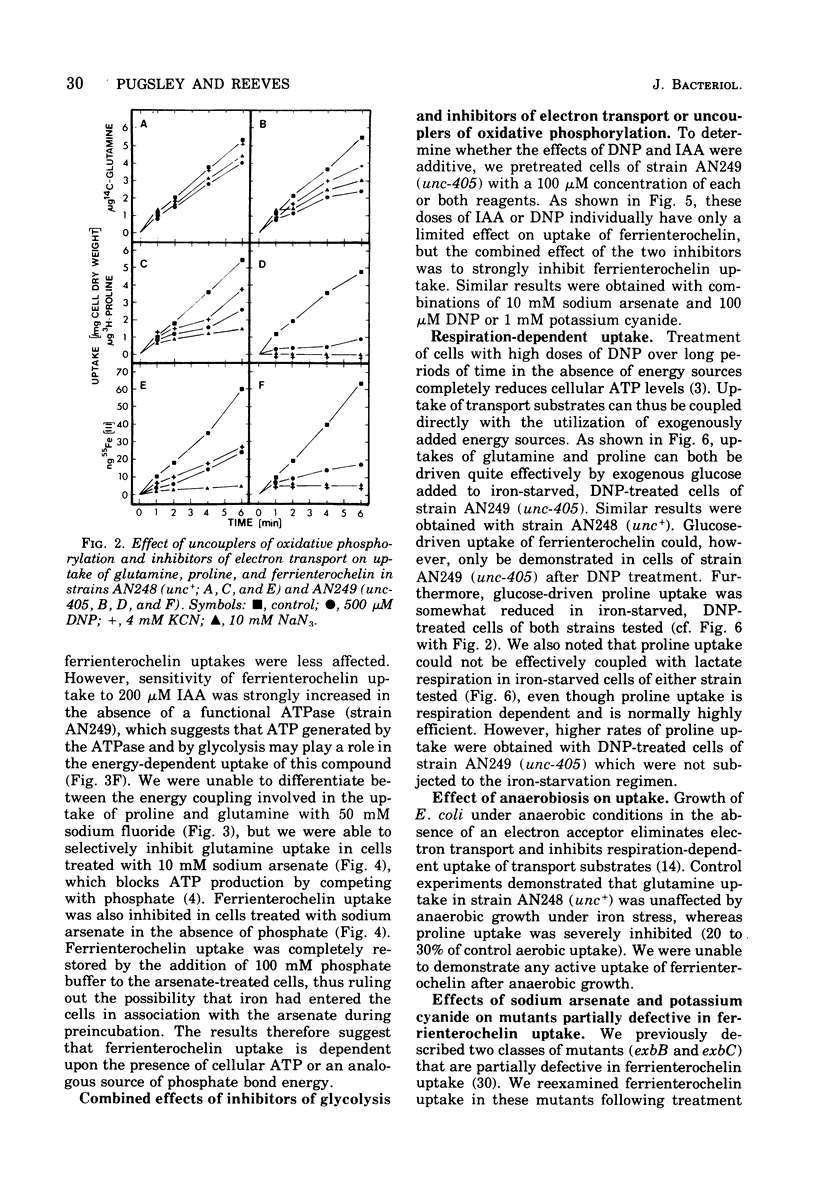
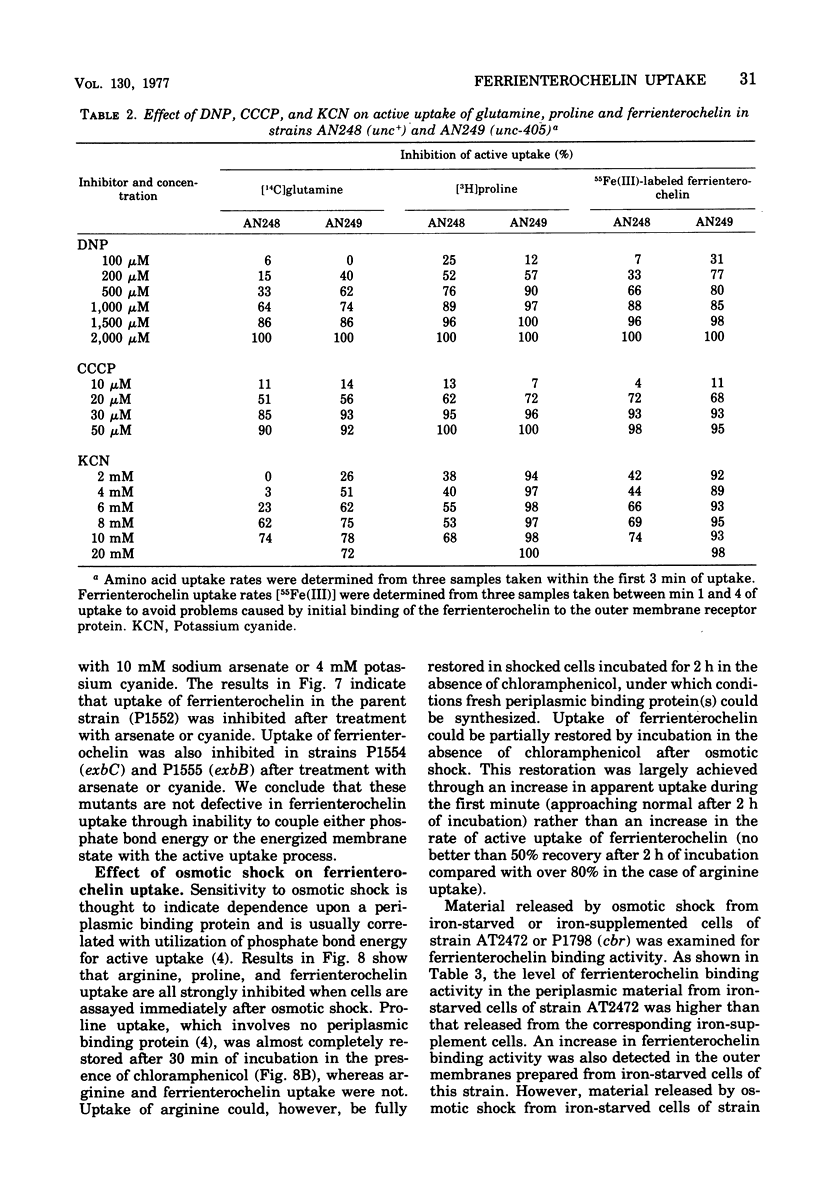
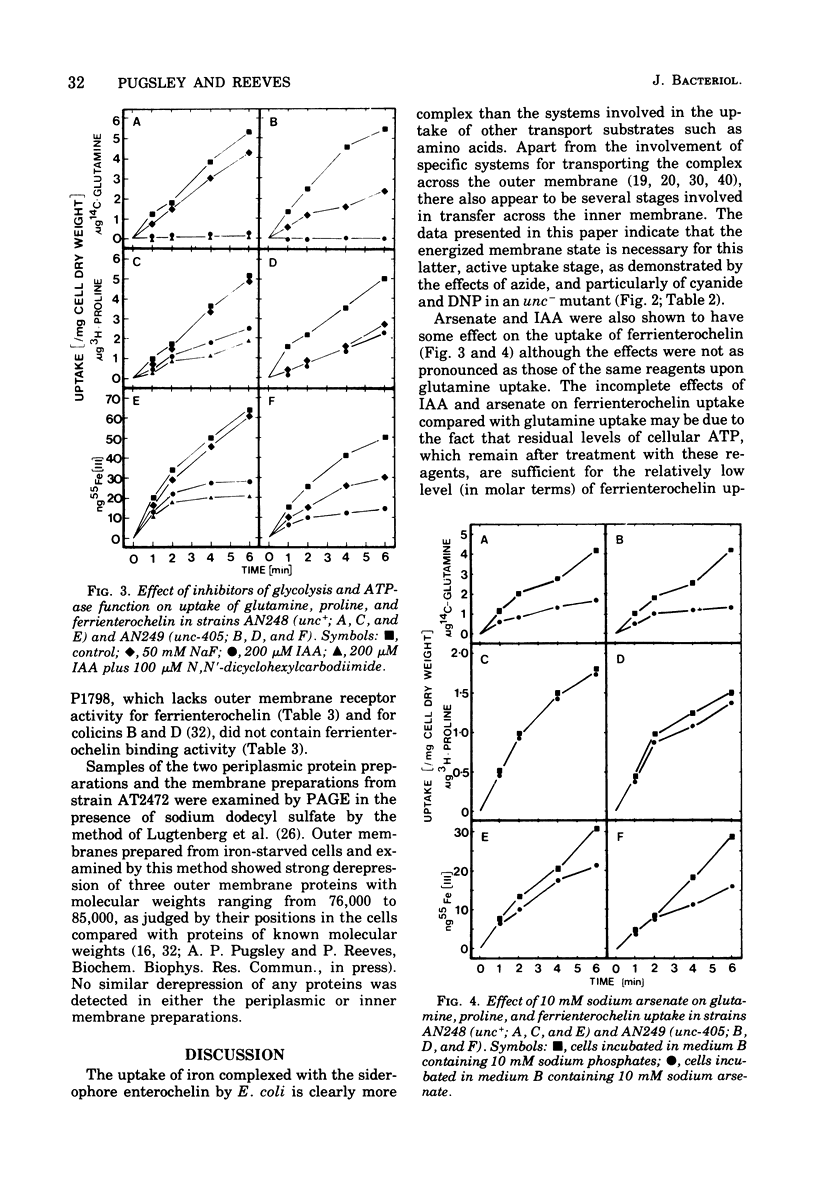
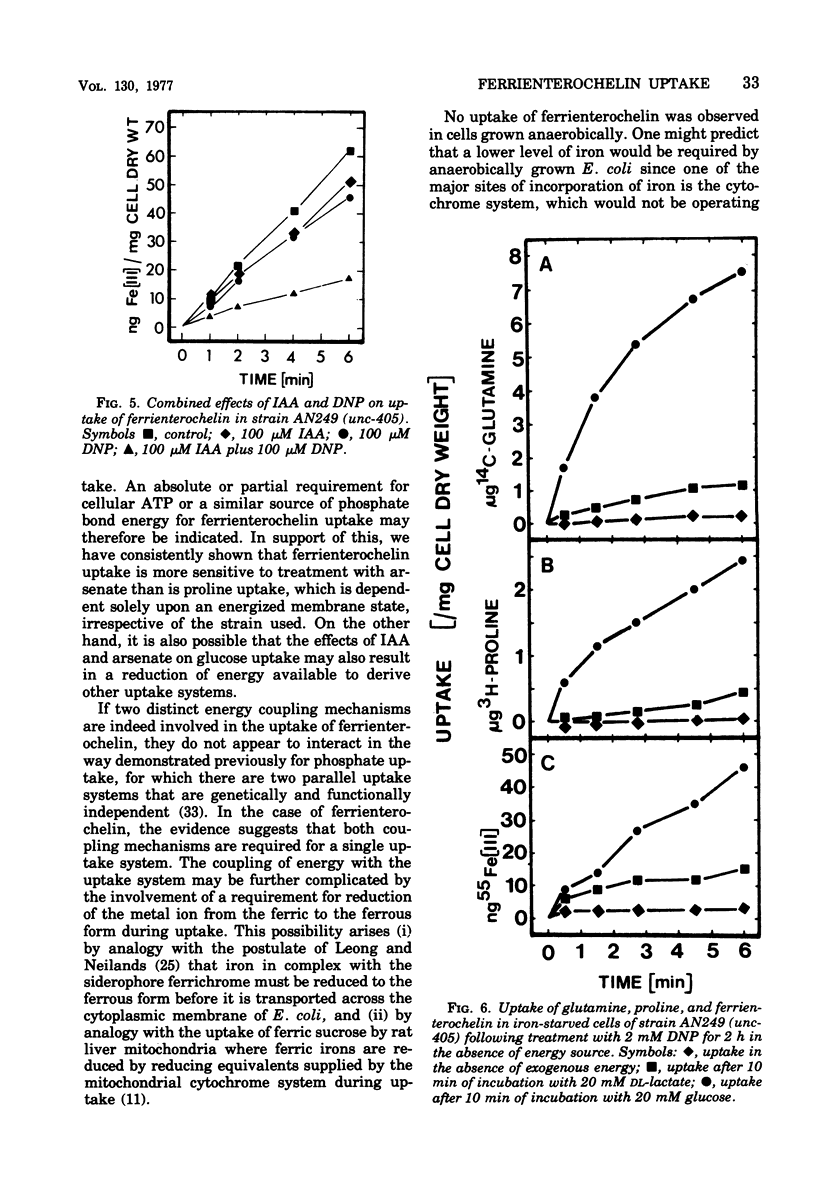
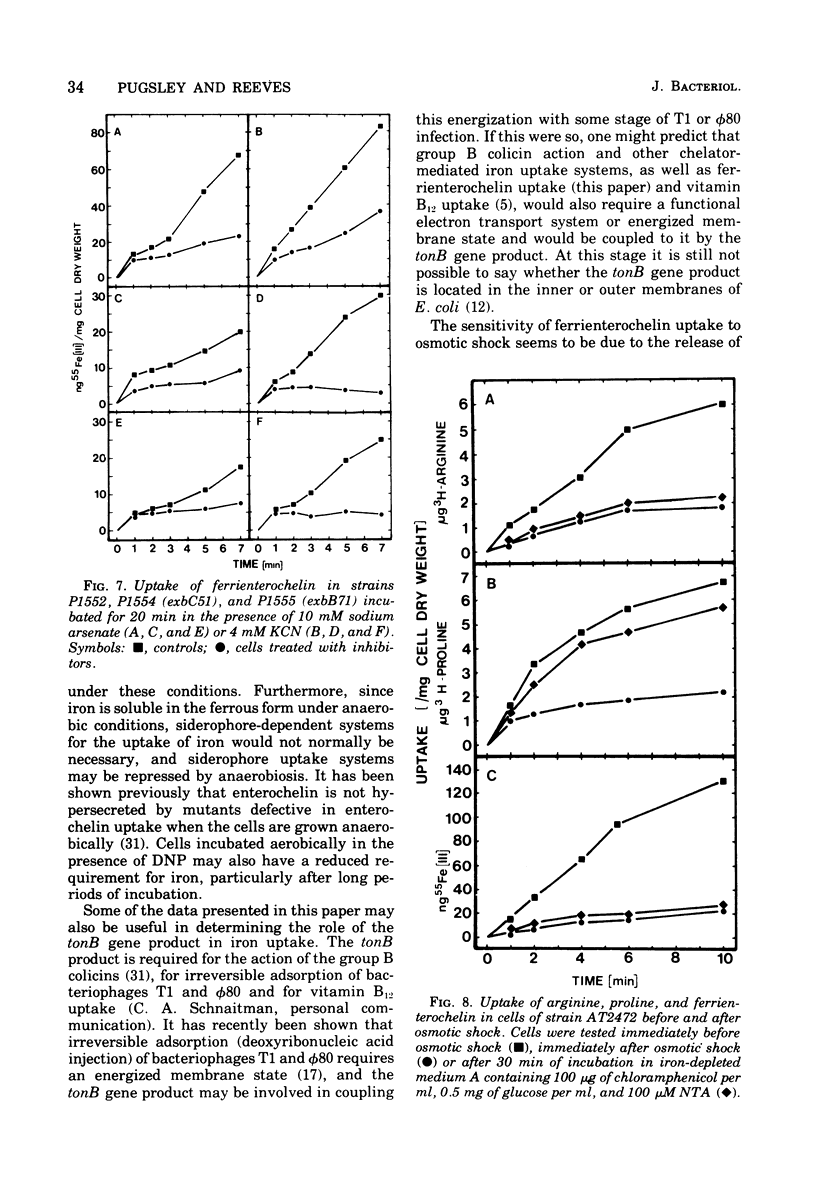
The uptake of the siderophore-iron complex ferrienterochelin was found to be strongly dependent upon an energized membrane state, as demonstrated by its sensitivity to dinitrophenol, azide, and cyanide. Ferrienterochelin uptake may also be dependent upon phosphate bond energy, as indicated by sensitivity to arsenate and iodoacetic acid. Although the adenosine triphosphatase does not appear to be involved in this energy coupling mechanism, ferrienterochelin uptake was shown to be less dependent upon phosphate bond energy than was glutamine uptake. Sensitivity of ferrienterochelin uptake to osmotic shock was shown to be due to the release of a ferrienterochelin binding compound located in the outer membrane of the cells and probably identical to the colicin B receptor protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Zitzmann W. Identification of the DCCD-reactive protein of the energy transducing adenosinetriphosphatase complex from Escherichia coli. FEBS Lett. 1975 Nov 15;59(2):268–272. doi: 10.1016/0014-5793(75)80390-5. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Bradbeer C., Woodrow M. L. Transport of vitamin B12 in Escherichia coli: energy dependence. J Bacteriol. 1976 Oct;128(1):99–104. doi: 10.1128/jb.128.1.99-104.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. Oxidative phosphorylation in Escherichia coli K12. An uncoupled mutant with altered membrane structure. Biochem J. 1974 Feb;138(2):211–215. doi: 10.1042/bj1380211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Flatmark T., Romslo I. Energy-dependent accumulation of iron by isolated rat liver mitochondria. Requirement of reducing equivalents and evidence for a unidirectional flux of Fe(II) across the inner membrane. J Biol Chem. 1975 Aug 25;250(16):6433–6438. [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong C. E., Weiner J. H. Purification of a leucine-specific binding protein from Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1076–1083. doi: 10.1016/0006-291x(70)90349-9. [DOI] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Energy coupling to active transport in anaerobically grown mutants of Escherichia Coli K12. Biochem J. 1976 Mar 15;154(3):731–734. doi: 10.1042/bj1540731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Braun V. The colicin I receptor of Escherichia coli K-12 has a role in enterochelin-mediated iron transport. FEBS Lett. 1976 Jun 1;65(2):208–210. doi: 10.1016/0014-5793(76)80481-4. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. A function common to iron-enterochelin transport and action of colicins B, I, V in Escherichia coli. FEBS Lett. 1975 Nov 15;59(2):277–281. doi: 10.1016/0014-5793(75)80392-9. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L. The binding of maltose to 'virgin' maltose-binding protein is biphasic. Eur J Biochem. 1975 Dec 15;60(2):445–449. doi: 10.1111/j.1432-1033.1975.tb21022.x. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Energy coupling for methionine transport in Escherichia coli. J Bacteriol. 1975 Sep;123(3):985–991. doi: 10.1128/jb.123.3.985-991.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976 Jul;127(1):218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Increased production of the outer membrane receptors for colicins B, D and M by Escherichia coli under iron starvation. Biochem Biophys Res Commun. 1976 Jun 7;70(3):846–853. doi: 10.1016/0006-291x(76)90669-0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Iron uptake in colicin B-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1052–1062. doi: 10.1128/jb.126.3.1052-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae A. S., Strickland K. P., Medveczky N., Rosenberg H. Studies of phosphate transport in Escherichia coli. I. Reexamination of the effect of osmotic and cold shock on phosphate uptake and some attempts to restore uptake with phosphate binding protein. Biochim Biophys Acta. 1976 May 21;433(3):555–563. doi: 10.1016/0005-2736(76)90281-9. [DOI] [PubMed] [Google Scholar]
- Rae A. S., Strickland K. P. Studies on phosphate transport in Escherichia coli. II. Effects of metabolic inhibitors and divalent cations. Biochim Biophys Acta. 1976 May 21;433(3):564–582. doi: 10.1016/0005-2736(76)90282-0. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. II. Purification and properties of an arginine-specific binding protein. J Biol Chem. 1973 Feb 25;248(4):1211–1218. [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. C., DiGirolamo P. M., Fu M. L., Preston Y. A., Bradbeer C. Transport of vitamin B 12 in Escherichia coli. Location and properties of the initial B 12 -binding site. J Biol Chem. 1973 Jun 10;248(11):3978–3986. [PubMed] [Google Scholar]



