Abstract
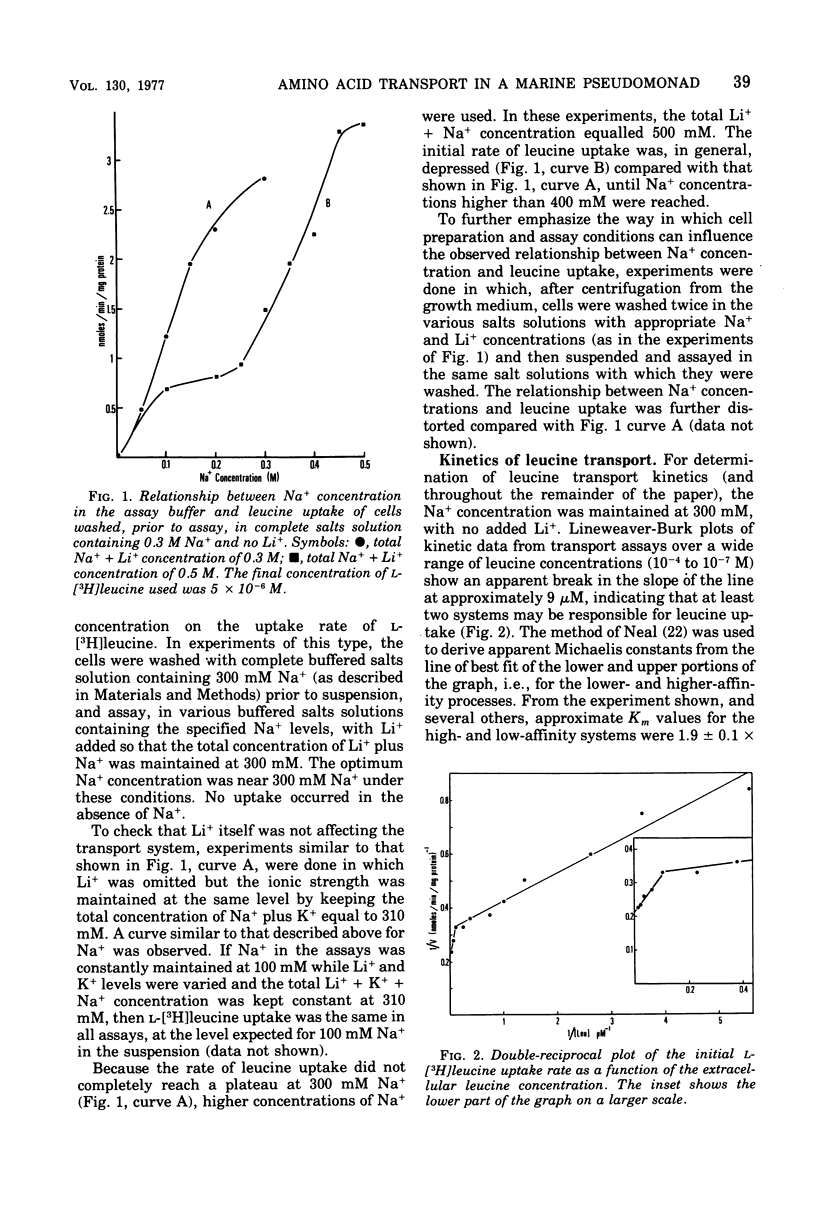
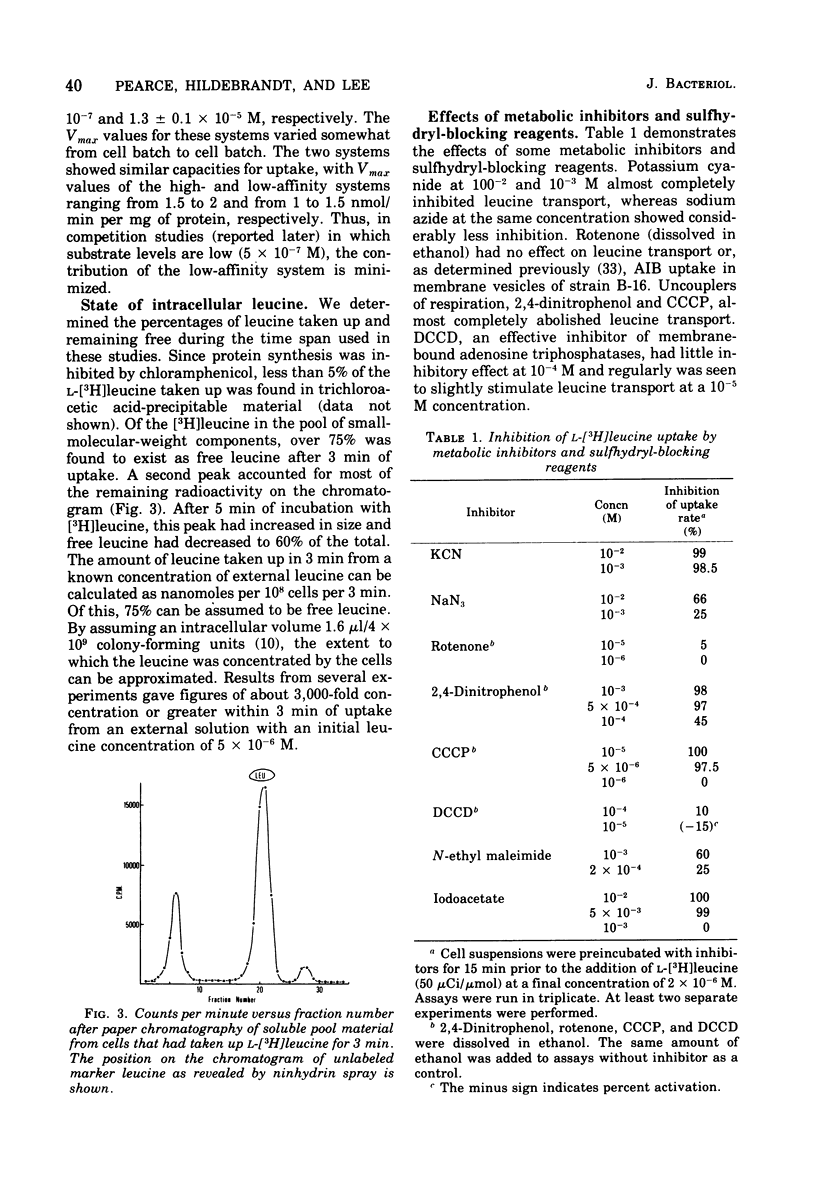
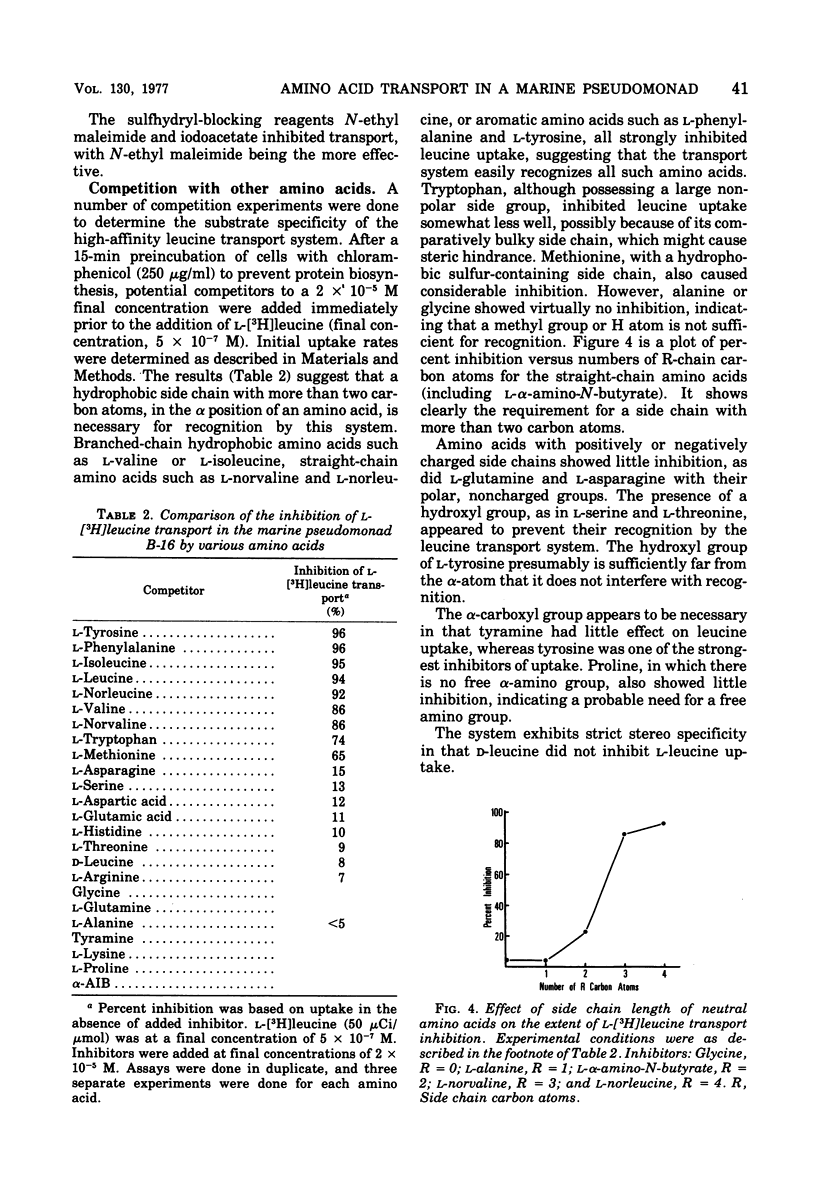
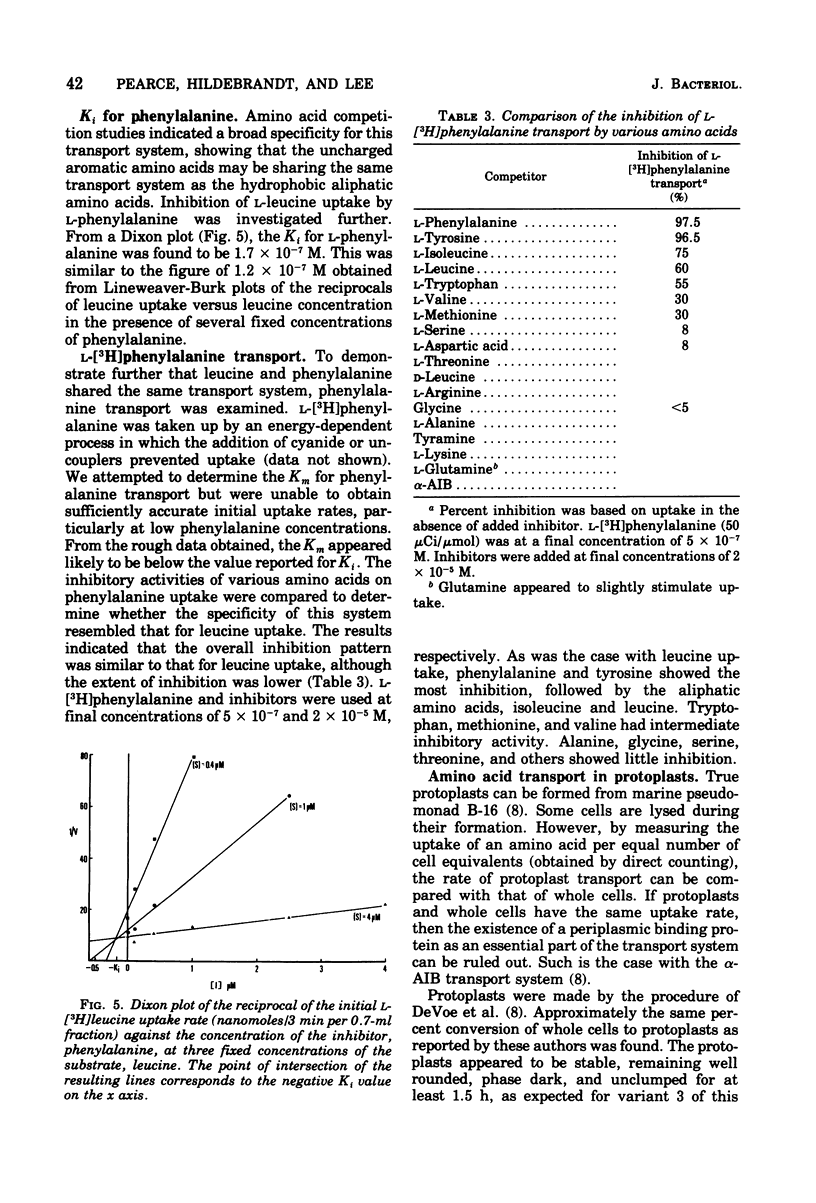
Uptake of leucine by the marine pseudomonad B-16 is an energy-dependent, concentrative process. Respiratory inhibitors, uncouplers, and sulfhydryl reagents block transport. The uptake of leucine is Na+ dependent, although the relationship between the rate of leucine uptake and Na+ concentration depends, to some extent, on the ionic strength of the suspending assay medium and the manner in which cells are washed prior to assay. Leucine transport can be separated into at least two systems: a low-affinity system with an apparent Km of 1.3 X 10(-5) M, and a high-affinity system with an apparent Km of 1.9 X 10(-7) M. The high-affinity system shows a specificity unusual for bacterial systems in that both aromatic and aliphatic amino acids inhibit leucine transport, provided that they have hydrophobic side chains of a length greater than that of two carbon atoms. The system exhibits strict stereospecificity for the L form. Phenylalanine inhibition was investigated in more detail. The Ki for inhibition of leucine transport by phenylalanine is about 1.4 X 10(-7) M. Phenylalanine itself is transported by an energy-dependent process whose specificity is the same as the high-affinity leucine transport system, as is expected if both amino acids share the same transport system. Studies with protoplasts indicate that a periplasmic binding protein is not an essential part of this transport system. Fein and MacLeod (J. Bacteriol. 124:1177-1190, 1975) reported two neutral amino acid transport systems in strain B-16: the DAG system, serving glycine, D-alanine, D-serine, and alpha-aminoisobutyric acid; and the LIV system, serving L-leucine, L-isoleucine, L-valine, and L-alanine. The high-affinity system reported here is a third neutral amino acid transport system in this marine pseudomonad. We propose the name "LIV-II" system.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reconstitution of energy-dependent transhydrogenase in ATPase-negative mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Feb 5;50(3):729–736. doi: 10.1016/0006-291x(73)91305-3. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- D'Ambrosio S. M., Glover G. I., Nelson S. O., Jensen R. A. Specificity of the tyrosine-phenylalanine transport system in Bacillus subtilis. J Bacteriol. 1973 Aug;115(2):673–681. doi: 10.1128/jb.115.2.673-681.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Voe I. W., Thompson J., Costerton J. W., MacLeod R. A. Stability and comparative transport capacity of cells, mureinoplasts, and true protoplasts of a gram-negative bacterium. J Bacteriol. 1970 Mar;101(3):1014–1026. doi: 10.1128/jb.101.3.1014-1026.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., MacLeod R. A. Characterization of neutral amino acid transport in a marine pseudomonad. J Bacteriol. 1975 Dec;124(3):1177–1190. doi: 10.1128/jb.124.3.1177-1190.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow J. A., DeVoe U. W., MacLeod R. A. Dissociation in a marine pseudomonad. Can J Microbiol. 1973 Jun;19(6):695–701. doi: 10.1139/m73-113. [DOI] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Multiplicity of isoleucine, leucine, and valine transport systems in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):382–392. doi: 10.1128/jb.117.2.382-392.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H. O., COHEN G. N. Incorporation des amino-acides endogènes et exogènes dans les protéines de la levure. Ann Inst Pasteur (Paris) 1958 Jul;95(1):73–87. [PubMed] [Google Scholar]
- Halpern Y. S. Genetics of amino acid transport in bacteria. Annu Rev Genet. 1974;8:103–133. doi: 10.1146/annurev.ge.08.120174.000535. [DOI] [PubMed] [Google Scholar]
- Kahane S., Marcus M., Barash H., Halpern Y. S. Sodium-dependent glutamate transport in membrane vesicles of Escherichia coli K-12. FEBS Lett. 1975 Aug 15;56(2):235–239. doi: 10.1016/0014-5793(75)81099-4. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):273–281. doi: 10.1128/jb.97.1.273-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Transport of aromatic amino acids by Pseudomonas aeruginosa. J Bacteriol. 1971 Mar;105(3):1039–1046. doi: 10.1128/jb.105.3.1039-1046.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACLEOD R. A. THE QUESTION OF THE EXISTENCE OF SPECIFIC MARINE BACTERIA. Bacteriol Rev. 1965 Mar;29:9–24. [PMC free article] [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport. Annu Rev Biochem. 1972;41(10):777–814. doi: 10.1146/annurev.bi.41.070172.004021. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. I. Properties of two amino acid transport systems. Biochim Biophys Acta. 1969 Jan 28;173(1):113–127. doi: 10.1016/0005-2736(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart C. A., Hubbard J. S. Energy coupling in the active transport of proline and glutamate by the photosynthetic halophile Ectothiorhodospira halophila. J Bacteriol. 1976 Sep;127(3):1255–1264. doi: 10.1128/jb.127.3.1255-1264.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Adler L. W. The maintenance of the energized membrane state and its relation to active transport in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):23–36. doi: 10.1016/0005-2728(75)90049-3. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C., Kaback H. R. Active transport in isolated bacterial membrane vesicles. V. The transport of amino acids by membrane vesicles prepared from Staphylococcus aureus. J Biol Chem. 1972 Jan 10;247(1):298–304. [PubMed] [Google Scholar]
- Sprott G. D., Drozdowski J. P., Martin E. L., MacLeod R. A. Kinetics of Naplus-dependent amino acid transport using cells and membrane vesicles of a marine pseudomonad. Can J Microbiol. 1975 Jan;21(1):43–50. doi: 10.1139/m75-006. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., MacLeod R. A. Nature of the specificity of alcohol coupling to L-alanine transport into isolated membrane vesicles of a marine pseudomonad. J Bacteriol. 1974 Mar;117(3):1043–1054. doi: 10.1128/jb.117.3.1043-1054.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton B. A., Savageau M. A. Transport of biosynthetic intermediates: homoserine and threonine uptake in Escherichia coli. J Bacteriol. 1974 Mar;117(3):1002–1009. doi: 10.1128/jb.117.3.1002-1009.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Na+ and K+ gradients and alpha-aminoisobutyric acid transport in a marine pseudomonad. J Biol Chem. 1973 Oct 25;248(20):7106–7111. [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Specific electron donor-energized transport of alpha-aminoisobutyric acid and K+ into intact cells of a marine pseudomonad. J Bacteriol. 1974 Mar;117(3):1055–1064. doi: 10.1128/jb.117.3.1055-1064.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. I. Specificity and kinetics. J Bacteriol. 1966 Dec;92(6):1698–1705. doi: 10.1128/jb.92.6.1698-1705.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R. Tryptophan transport in Neurospora crassa: a tryptophan-binding protein released by cold osmotic shock. J Bacteriol. 1970 Sep;103(3):656–662. doi: 10.1128/jb.103.3.656-662.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T., Thompson J., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVII. Ion-dependent retention of alpha-aminoisobutyric acid and its relation to Na+ dependent transport in a marine pseudomonad. J Biol Chem. 1969 Feb 10;244(3):1016–1025. [PubMed] [Google Scholar]


