Abstract
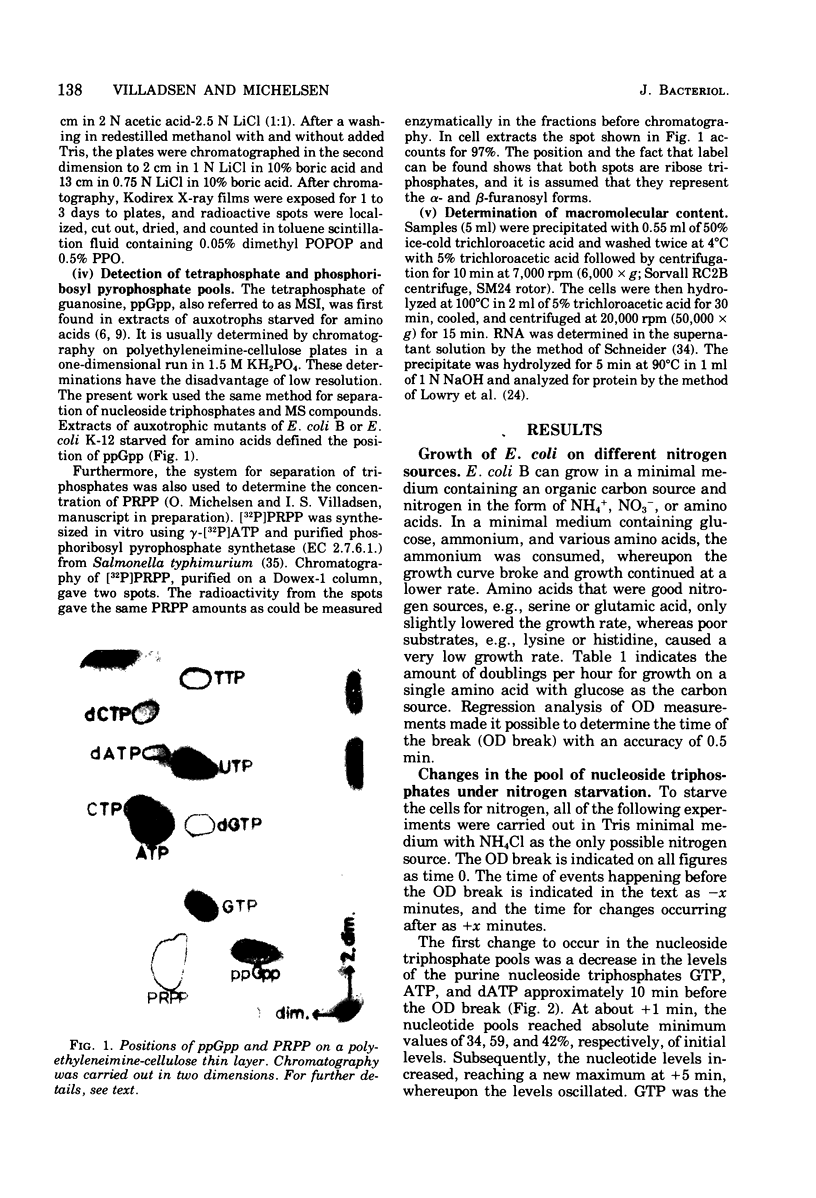
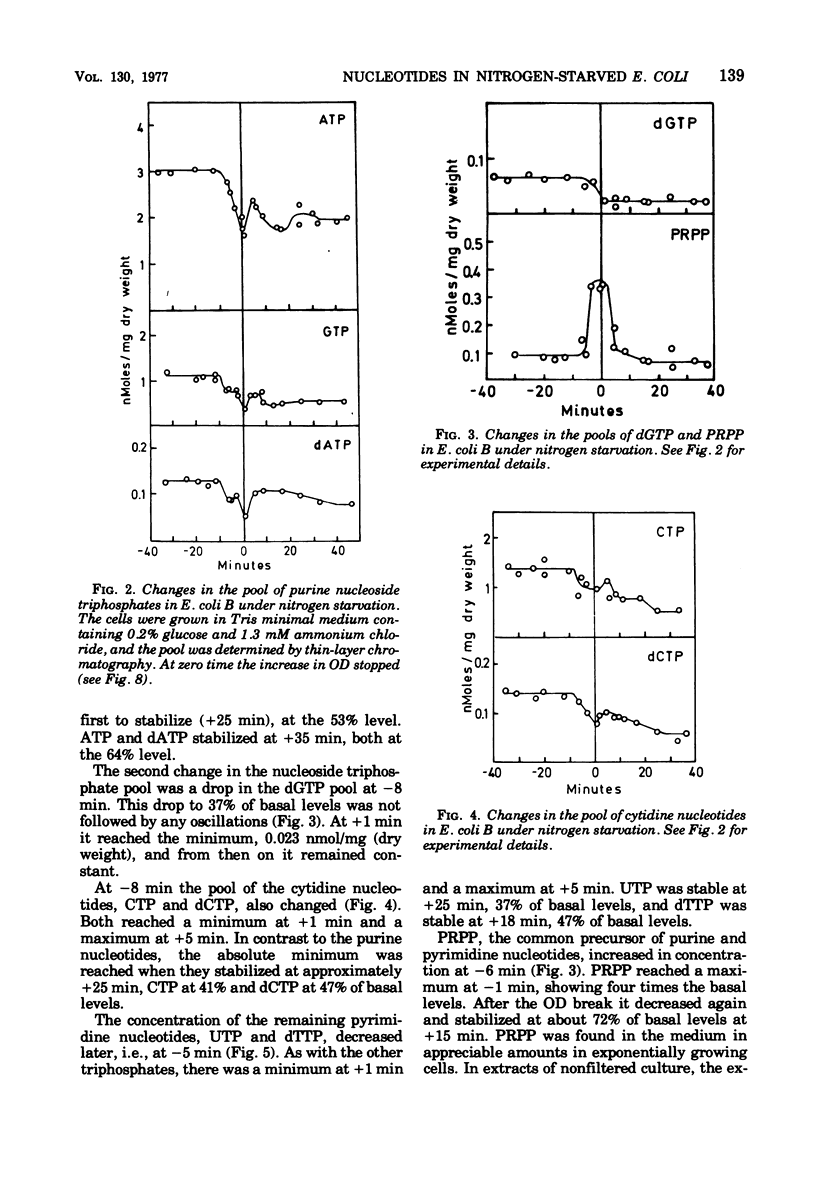
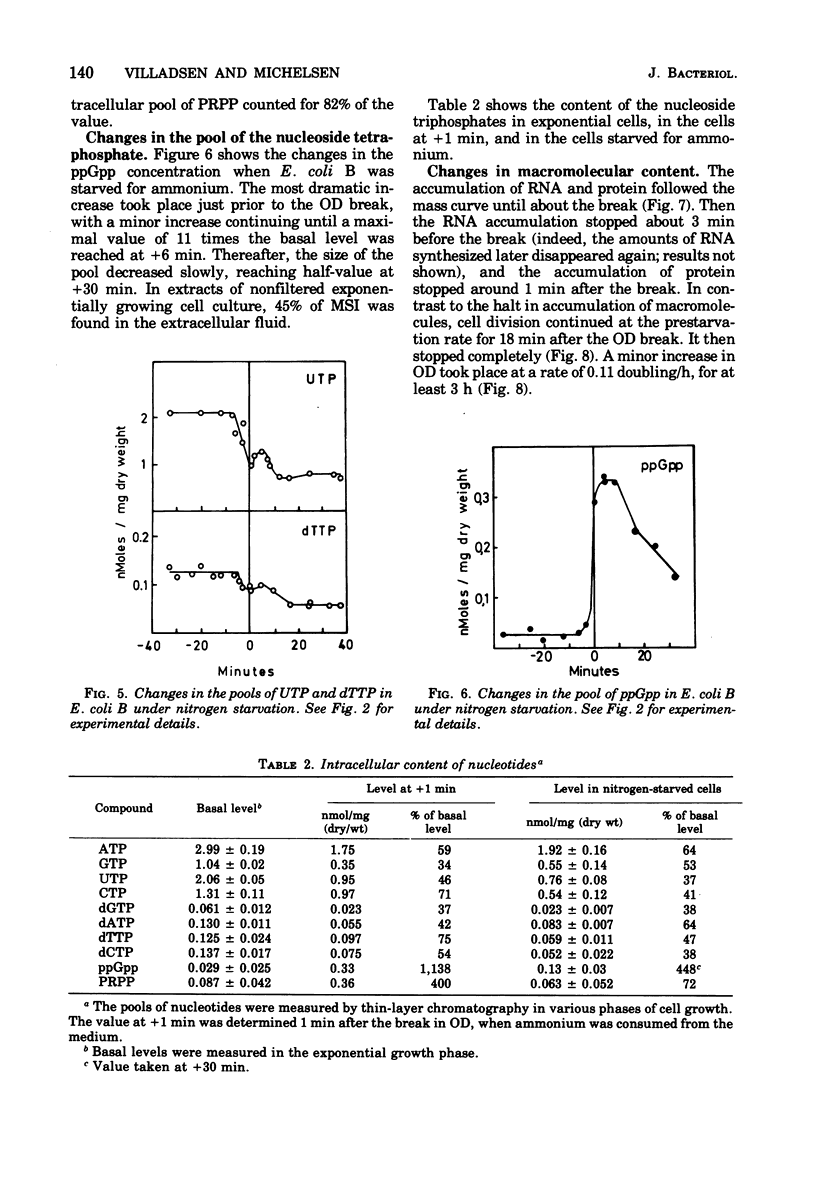
The ribonucleoside triphosphate, deoxyribonucleoside triphosphate, 3' -diphosphate guanosine 5' -diphosphate (ppGpp), and 5-phosphoribosyl 1-pyrophosphate (PRPP) pools in Escherichia coli B were determined by thin-layer chromatography during changing conditions to ammonium starvation. The intracellular concentrations of all nucleotides were found to change in a well-defined order several minutes before andy observed change in the optical density of the culture. The levels of purine nucleoside triphosphates (adenosine 5' -triphosphate [CTP], dCTP) and uridine nucleotides (uridine 5' -triphosphate, deoxythymidine 5'-triphosphate). The deoxyribonucleotides thus behaved as the ribonucleotides. The levels of ppGpp increased 11-fold after the decrease in uridine nucleotides, when the accumulation of stable ribonucleic acid (RNA) stopped. The level of the nucleotide pool did not stabilize until 30 min after the change in optical density. The pool of dGTP dropped concomitantly with the pool of CTP. The nucleotide precursor PRPP exhibited a transient increase, wtih maximum value of four times the exponential levels at the onset of starvation. Apparently the cell adjusts early to starvation by reducing either the phosphorylating activity or the nucleotide biosynthetic activity. As in other downshift systems, the accumulation of stable RNA stopped before the break in optical density and before the stop in protein accumulation. Cell divisions were quite insensitive to the control mechanisms operating on RNA and protein accumulation under ammonium starvation, since the cells continued to divide for 21 min without any net accumulation of RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnara A. S., Finch L. R. Relationships between intracellular contents of nucleotides and 5-phosphoribosyl 1-pyrophosphate in Escherichia coli. Eur J Biochem. 1973 Jul 16;36(2):422–427. doi: 10.1111/j.1432-1033.1973.tb02927.x. [DOI] [PubMed] [Google Scholar]
- Beck C., Ingraham J., Maaloe O., Neuhard J. Relationship between the concentration of nucleoside triphosphates and the rate of synthesis of RNA. J Mol Biol. 1973 Jun 25;78(1):117–121. doi: 10.1016/0022-2836(73)90431-2. [DOI] [PubMed] [Google Scholar]
- Ben-Hamida F., Schlessinger D. Synthesis and breakdown of ribonucleic acid in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):183–191. doi: 10.1016/0005-2787(66)90049-9. [DOI] [PubMed] [Google Scholar]
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Brenchley J. E., Prival M. J., Magasanik B. Regulation of the synthesis of enzymes responsible for glutamate formation in Klebsiella aerogenes. J Biol Chem. 1973 Sep 10;248(17):6122–6128. [PubMed] [Google Scholar]
- Cashel M., Gallant J. Control of RNA synthesis in Escherichia coli. I. Amino acid dependence of the synthesis of the substrates of RNA polymerase. J Mol Biol. 1968 Jul 14;34(2):317–330. doi: 10.1016/0022-2836(68)90256-8. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Coote J. G., Hassall H. The control of the enzymes degrading histidine and related imidazolyl derivates in Pseudomonas testosteroni. Biochem J. 1973 Mar;132(3):423–433. doi: 10.1042/bj1320423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzler D. N., Lais C. J., Leckie M. P. Simultaneous increases of the adenylate energy charge and the rate of glycogen synthesis in nitrogen-starved Escherichia coli W4597(K). Arch Biochem Biophys. 1974 Jan;160(1):14–25. doi: 10.1016/s0003-9861(74)80003-2. [DOI] [PubMed] [Google Scholar]
- Edlin G., Donini P. Synthesis of guanosine 5'-diphosphate, 2'-(or 3'-) diphosphate and related nucleotides in a variety of physiological conditions. J Biol Chem. 1971 Jul 10;246(13):4371–4373. [PubMed] [Google Scholar]
- FRANZEN J. S., BINKLEY S. B. Comparison of the acid-soluble nucleotides in Escherichia coli at different growth rates. J Biol Chem. 1961 Feb;236:515–519. [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Irr J. D. Control of nucleotide metabolism and ribosomal ribonucleic acid synthesis during nitrogen starvation of Escherichia coli. J Bacteriol. 1972 May;110(2):554–561. doi: 10.1128/jb.110.2.554-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Effect of infection with T-even phage on the inducible synthesis of beta-glactosidase in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):453–468. doi: 10.1016/0022-2836(67)90051-4. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Deppe C. S. In vivo assay of protein synthesizing capacity of Escherichia coli from slowly growing chemostat cultures. J Mol Biol. 1971 Feb 14;55(3):549–562. doi: 10.1016/0022-2836(71)90336-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lazzarini R. A., Johnson L. D. Regulation of ribosomal RNA synthesis in cold-shocked E. coli. Nat New Biol. 1973 May 2;243(122):17–20. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Cashel M., Weissbach H. The interaction of guanosine 5'-diphosphate, 2' (3')-diphosphate with the bacterial elongation factor Tu. Arch Biochem Biophys. 1973 Feb;154(2):675–682. doi: 10.1016/0003-9861(73)90022-2. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Wong J. T. Nucleotide changes and the regulation of ribonucleic acid accumulation during growth rate shifts in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):790–797. [PubMed] [Google Scholar]
- Neuhard J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1519–1527. doi: 10.1128/jb.96.5.1519-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Randerath E., Randerath K. Ion-exchange thin-layer chromatography. 13. Resolution of complex nucleoside triphosphate mixtures. Anal Biochem. 1965 Nov;13(2):211–222. doi: 10.1016/0003-2697(65)90191-0. [DOI] [PubMed] [Google Scholar]
- Olszowy J., Switzer R. L. Specific repression of phosphoribosylpyrophosphate synthetase by uridine compounds in Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):450–451. doi: 10.1128/jb.110.1.450-451.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Schlessinger D., Ben-Hamida F. Turnover of protein in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):171–182. doi: 10.1016/0005-2787(66)90048-7. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. I. Purification and properties of the enzyme from Salmonella typhimurium. J Biol Chem. 1969 Jun 10;244(11):2854–2863. [PubMed] [Google Scholar]
- Tyler B., Deleo A. B., Magasanik B. Activation of transcription of hut DNA by glutamine synthetase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):225–229. doi: 10.1073/pnas.71.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Travers A., Clark B. F. Inhibition of translation initiation complex formation by MS1. FEBS Lett. 1972 Jun 15;23(2):163–166. doi: 10.1016/0014-5793(72)80331-4. [DOI] [PubMed] [Google Scholar]