Abstract
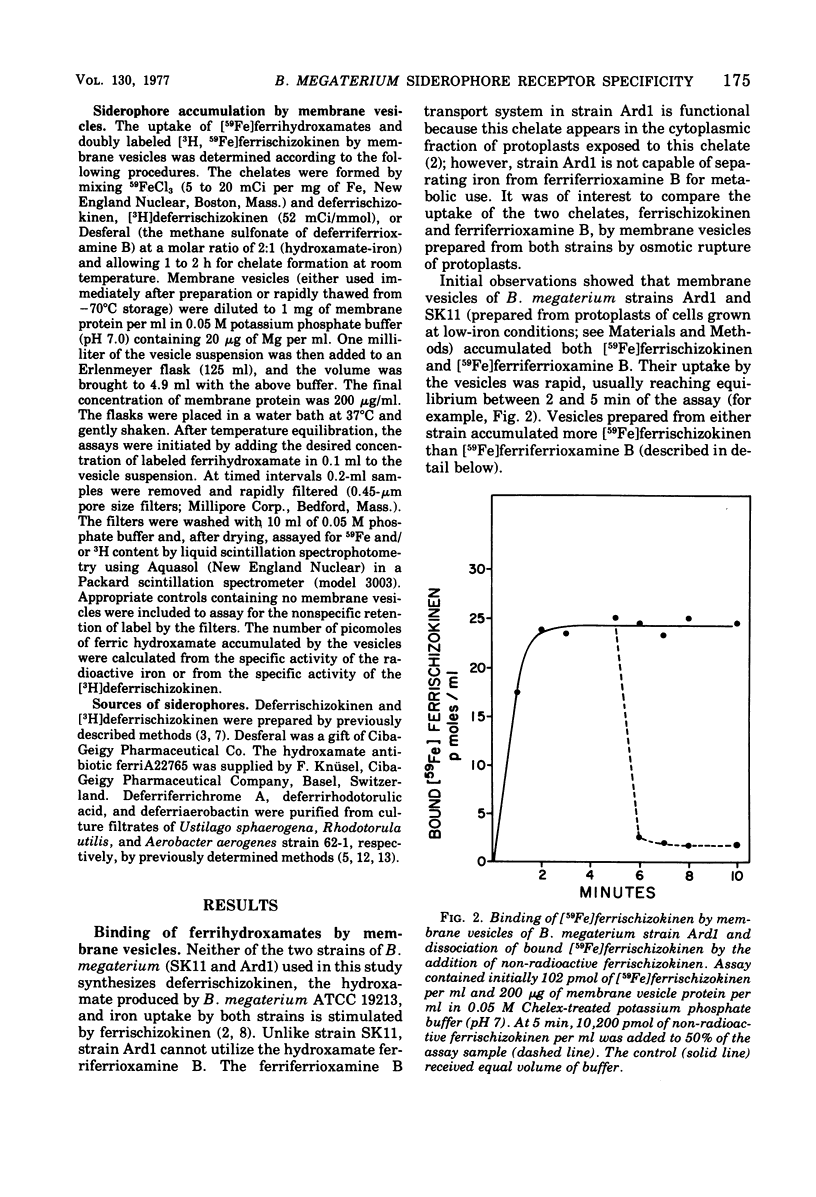
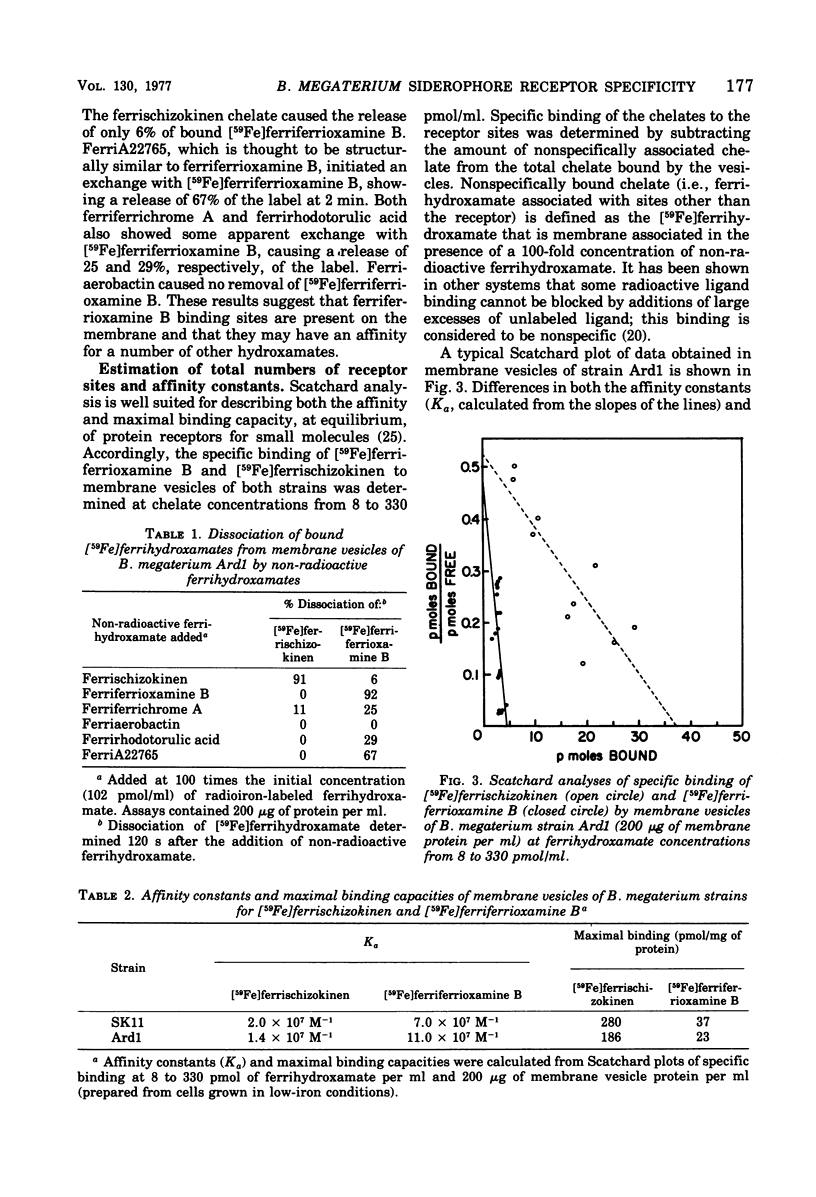
Membrane vesicles of Bacillus megaterium strains SK11 and Ard1 bound the ferrischizokinen and ferriferrioxamine B siderhores (iron transport cofactors). An approximately equimolar uptake of both labels of [3H, 59Fe]ferrischizokinen indicated binding of the intact chelate. Binding reached equilibrium in 2 to 5 min, was temperature independent, and was unaltered by the addition of several energy sources. A 91% dissociation of bound [Fe]ferrischizokinen was achieved in 60 s by the addition of excess ferrischizokinen. Ferriaerobactin, a siderophore which is structurally related to ferrischizokinen, caused no detectable release of bound [59Fe]ferrischizokinen. Of several other ferrigydroxamates tested, only ferriferrichrome A achieved the release (11%) of [Fe]ferrischizokinen. Rapid dissociation (92%) of bound [59Fe]ferriferrioxamine B by the addition of ferriferrioxamine B was observed, and a 67% release of [59Fe]ferriferrioxamine B was caused by ferriA2265, its structural relative. Ferrischizokinen, ferriferrichrome A, and ferrirhodotorulic acid produced a 6, 25, and 29% dissociation, respectively, of [59Fe]ferriferrioxamine B; ferriaerobactin caused no dissociation. [59Fe]ferriaerobactin was bound by the membranes, but its dissociation was not effected by unlabeled ferriaerobactin, suggesting no specific receptors for this chelate. The respective binding affinity constants and maximal binding capacities of membrane vesicles of strain SK11 were 2 x 10(7) M-1 and 280 pmol per mg of protein for ferrischizokinen and 7 x 10(7) M-1 and 37 pmol per mg of protein for ferriferrioxamine B. These values in strain Ard1 were, respectively, 1.4 x 10(7) M-1 and 186 pmol per mg of protein for ferrischizokinen and 11 x 10(7) M-1 and 23 pmol per mg of protein for ferriferrioxamine B. Separate, specific binding sites (receptors) for ferrischizokinen and ferriferrioxamine B exist on the vesicles. The ferrischizokinen receptors have a lower affinity but a higher binding capacity (eightfold) than that shown by the ferriferrioxamine B receptor. These receptors may be components of independent transport systems.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Arceneaux J. E., Byers B. R. Ferric hydroxamate transport without subsequent iron utilization in Bacillus megaterium. J Bacteriol. 1976 Sep;127(3):1324–1330. doi: 10.1128/jb.127.3.1324-1330.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arceneaux J. E., Davis W. B., Downer D. N., Haydon A. H., Byers B. R. Fate of labeled hydroxamates during iron transport from hydroxamate-ion chelates. J Bacteriol. 1973 Sep;115(3):919–927. doi: 10.1128/jb.115.3.919-927.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arceneaux J. L., Lankford C. E. A schizokinen (siderochrome) auxotroph of Bacillus megaterium induced with N-methyl-N'-nitro-N-nitrosoguanidine. Biochem Biophys Res Commun. 1966 Aug 12;24(3):370–375. doi: 10.1016/0006-291x(66)90166-5. [DOI] [PubMed] [Google Scholar]
- Atkin C. L., Neilands J. B. Rhodotorulic acid, a diketopiperazine dihydroxamic acid with growth-factor activity. I. Isolation and characterization. Biochemistry. 1968 Oct;7(10):3734–3739. doi: 10.1021/bi00850a054. [DOI] [PubMed] [Google Scholar]
- Byers B. R., Powell M. V., Lankford C. E. Iron-chelating hydroxamic acid (schizokinen) active in initiation of cell division in Bacillus megaterium. J Bacteriol. 1967 Jan;93(1):286–294. doi: 10.1128/jb.93.1.286-294.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. B., Byers B. R. Active transport of iron in Bacillus megaterium: role of secondary hydroxamic acids. J Bacteriol. 1971 Aug;107(2):491–498. doi: 10.1128/jb.107.2.491-498.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. B., McCauley M. J., Byers B. R. Iron requirements and aluminum sensitivity of an hydroxamic acid-requiring strain of Bacillus megaterium. J Bacteriol. 1971 Feb;105(2):589–594. doi: 10.1128/jb.105.2.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandes B., Frehel C., Chaix P. Fractionment et purification des systèmes membranaires cytoplasmiques et mésosomiquees de lbacillus subtilis. Etude de quelques-unes de leurs propríetés oxydo-réductricwa associées à la chaine respiratoire. Biochim Biophys Acta. 1970 Dec 8;223(2):292–308. doi: 10.1016/0005-2728(70)90186-6. [DOI] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport of Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J Bacteriol. 1976 Sep;127(3):1370–1375. doi: 10.1128/jb.127.3.1370-1375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Haydon A. H., Davis W. B., Arceneaux J. E., Byers B. R. Hydroxamate recognition during iron transport from hydroxamate-ion chelates. J Bacteriol. 1973 Sep;115(3):912–918. doi: 10.1128/jb.115.3.912-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luckey M., Neilands J. B. Iron transport in Salmonella typhimurium LT-2: prevention, by ferrichrome, of adsorption of bacteriophages ES18 and ES18.h1 to a common cell envelope receptor. J Bacteriol. 1976 Aug;127(2):1036–1037. doi: 10.1128/jb.127.2.1036-1037.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



