Abstract
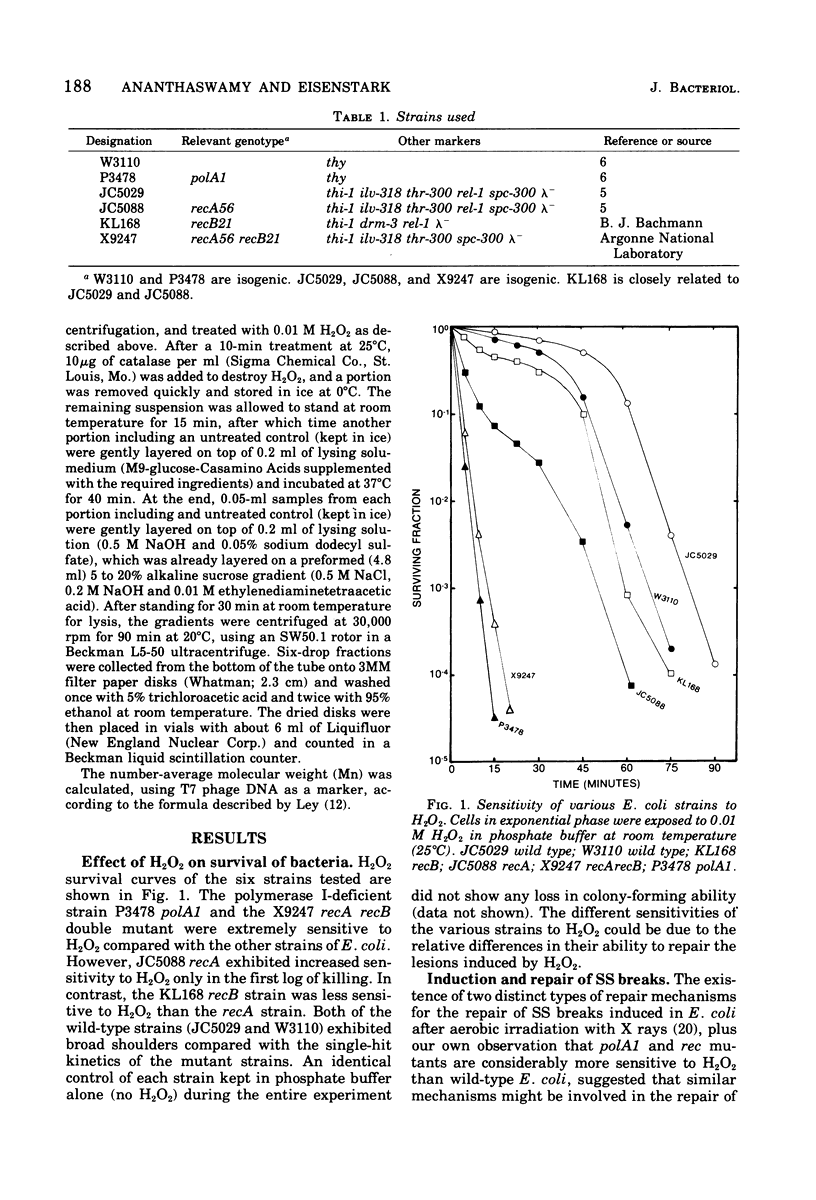
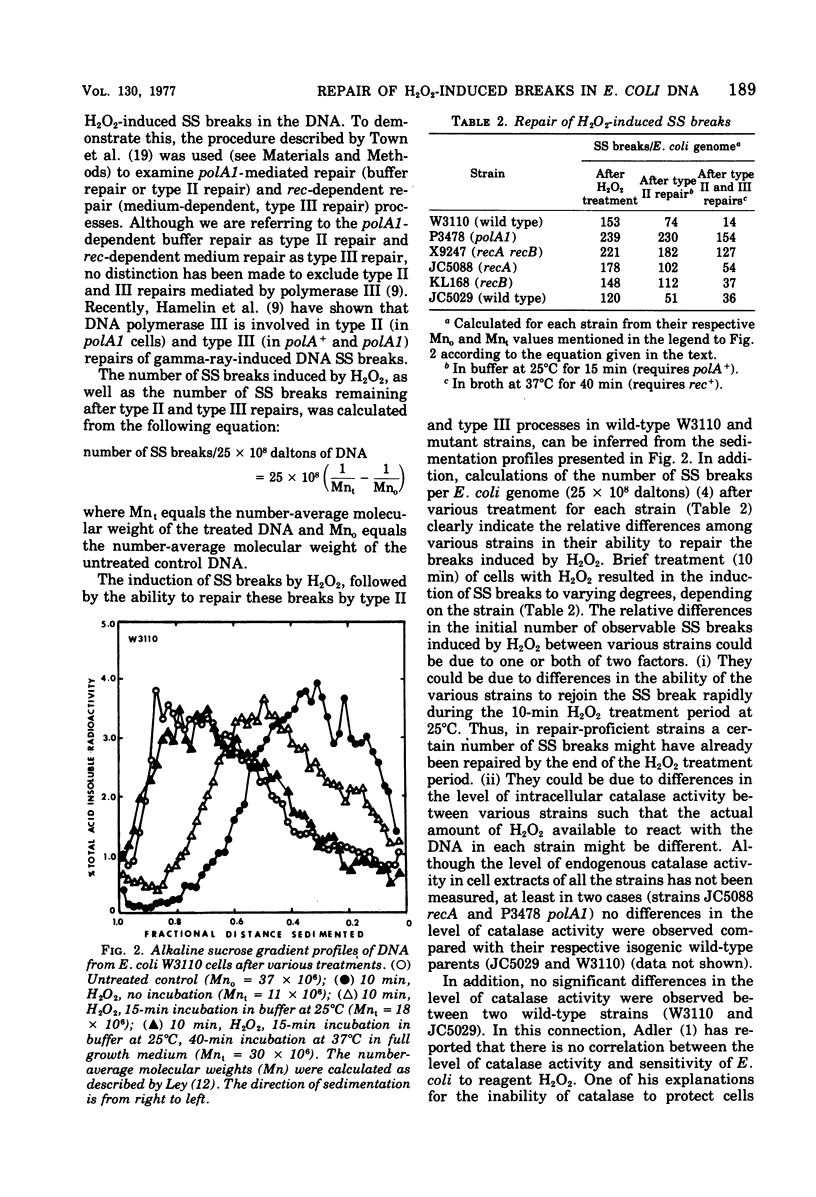
Near-ultraviolet (300 to 400 nm) irradiation of L-tryptophan yielded H2O2 (a toxic photoproduct) that was selectively lethal for rec and polA1 Escherichia coli mutants. H2O2 treatment of cells resulted in the induction of single-strand deoxyribonucleic acid breaks. These breaks were repaired to only a small extent in polA1, recA recB, and recA mutants, but were efficiently repaired in wild-type strains. We conclude that H2O2 deoxyribonucleic acid lesions require both the polA+ and recA+ pathways for repair.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I. Catalase, hydrogen peroxide, and ionizing radiation. Radiat Res. 1963;Suppl 3:110–129. [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Near-UV-induced breaks in phage DNA: sensitization by hydrogen peroxide (a tryptophan photoproduct). Photochem Photobiol. 1976 Nov;24(5):439–442. doi: 10.1111/j.1751-1097.1976.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Freese E. B., Gerson J., Taber H., Rhaese H. J., Freese E. Inactivating DNA alterations induced by peroxides and peroxide-producing agents. Mutat Res. 1967 Sep-Oct;4(5):517–531. doi: 10.1016/0027-5107(67)90038-3. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Donta S. T., Goldstein R. X-ray inactivation of bacteriophages: role of O 2 -dependent damage in single- and double-strand DNA phages. Virology. 1972 Nov;50(2):516–519. doi: 10.1016/0042-6822(72)90402-3. [DOI] [PubMed] [Google Scholar]
- Hamelin C., Youngs D. A., Smith K. C. Role of deoxyribonucleic acid polymerase III in the repair of single-strand breaks produced in Escherichia coli deoxyribonucleic acid by gamma radiation. J Bacteriol. 1976 Sep;127(3):1307–1314. doi: 10.1128/jb.127.3.1307-1314.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation and repair of gamma-ray induced thymine damage in Micrococcus radiodurans. J Mol Biol. 1972 Apr 28;66(1):65–81. doi: 10.1016/s0022-2836(72)80006-8. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. D. Postreplication repair in an excision-defective mutant of Escherichia coli: ultraviolet light-induced incorporation of bromodeoxyuridine into parental DNA. Photochem Photobiol. 1973 Aug;18(2):87–95. doi: 10.1111/j.1751-1097.1973.tb06397.x. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Ginoza W. Frequency of single-strand breaks per lethal gamma-ray hit in phi-X 174 DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Jan 17;14(6):553–560. doi: 10.1080/09553006914551721. [DOI] [PubMed] [Google Scholar]
- McCormick J. P., Fischer J. R., Pachlatko J. P., Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science. 1976 Feb 6;191(4226):468–469. doi: 10.1126/science.1108203. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Rhaese H. J., Freese E. Chemical analysis of DNA alterations. I. Base liberation and backbone breakage of DNA and oligodeoxyadenylic acid induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968 Feb 26;155(2):476–490. [PubMed] [Google Scholar]
- Taylor W. D., Ginoza W. Correlation of gamma-ray inactivation and strand scission in the replicative form of phi-X174 bacteriophage DNA. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1753–1757. doi: 10.1073/pnas.58.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Repair of X-ray damage to bacterial DNA. Curr Top Radiat Res Q. 1973 Jul;8(4):351–399. [PubMed] [Google Scholar]
- Uchida Y., Shigematu H., Yamafuji K. The mode of action of hydrogen peroxide on deoxyribonucleic acid. Enzymologia. 1965 Dec 31;29(6):369–376. [PubMed] [Google Scholar]
- Yamafuji K., Uchida Y. Liberation of adenine from deoxyribonucleic acid by hydrogen peroxide. Nature. 1966 Jan 15;209(5020):301–302. doi: 10.1038/209301a0. [DOI] [PubMed] [Google Scholar]
- Yoakum G., Eisenstark A. Toxicity of L-Tryptophan photoproduct on recombinationless (rec) mutants of Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):653–655. doi: 10.1128/jb.112.1.653-655.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schans G. P., Bleichrodt J. F., Blok J. Contribution of various types of damage to inactivation of a biologically-active double-stranded circular DNA by gamma-radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Feb;23(2):133–150. doi: 10.1080/09553007314550151. [DOI] [PubMed] [Google Scholar]



