Abstract
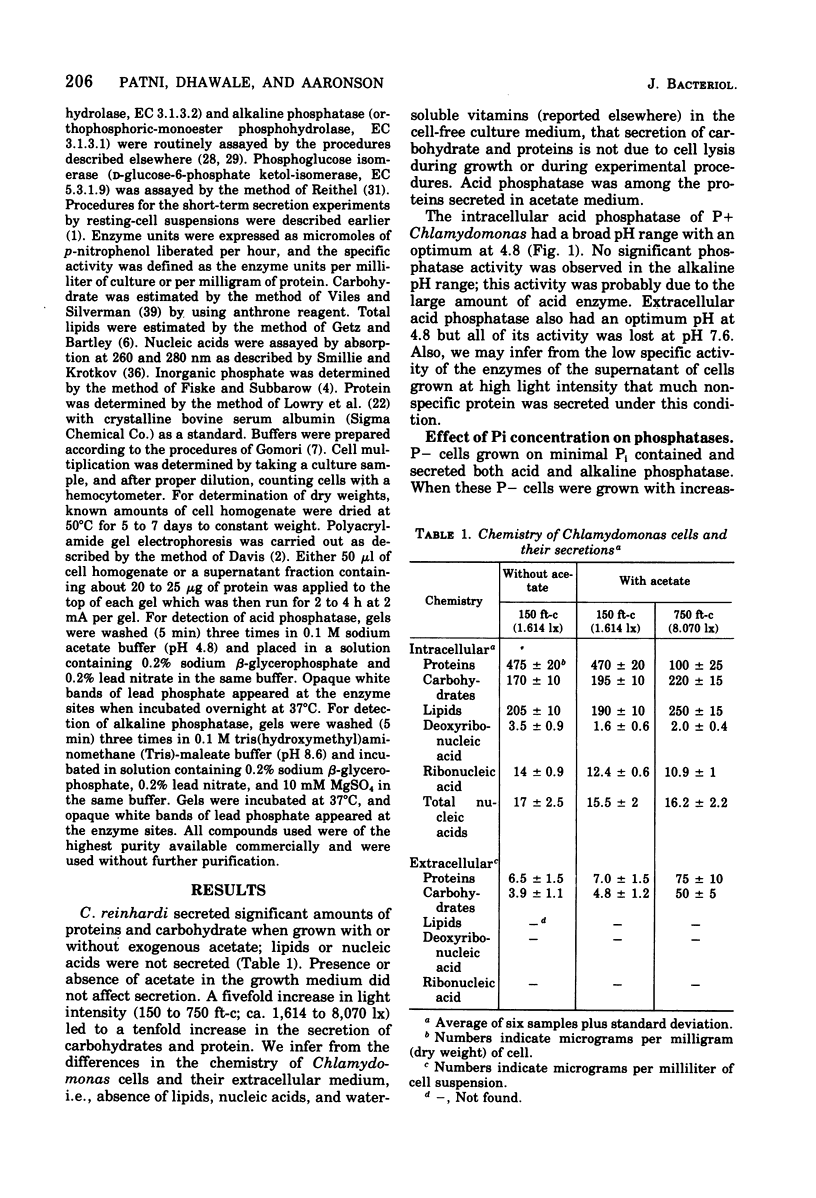
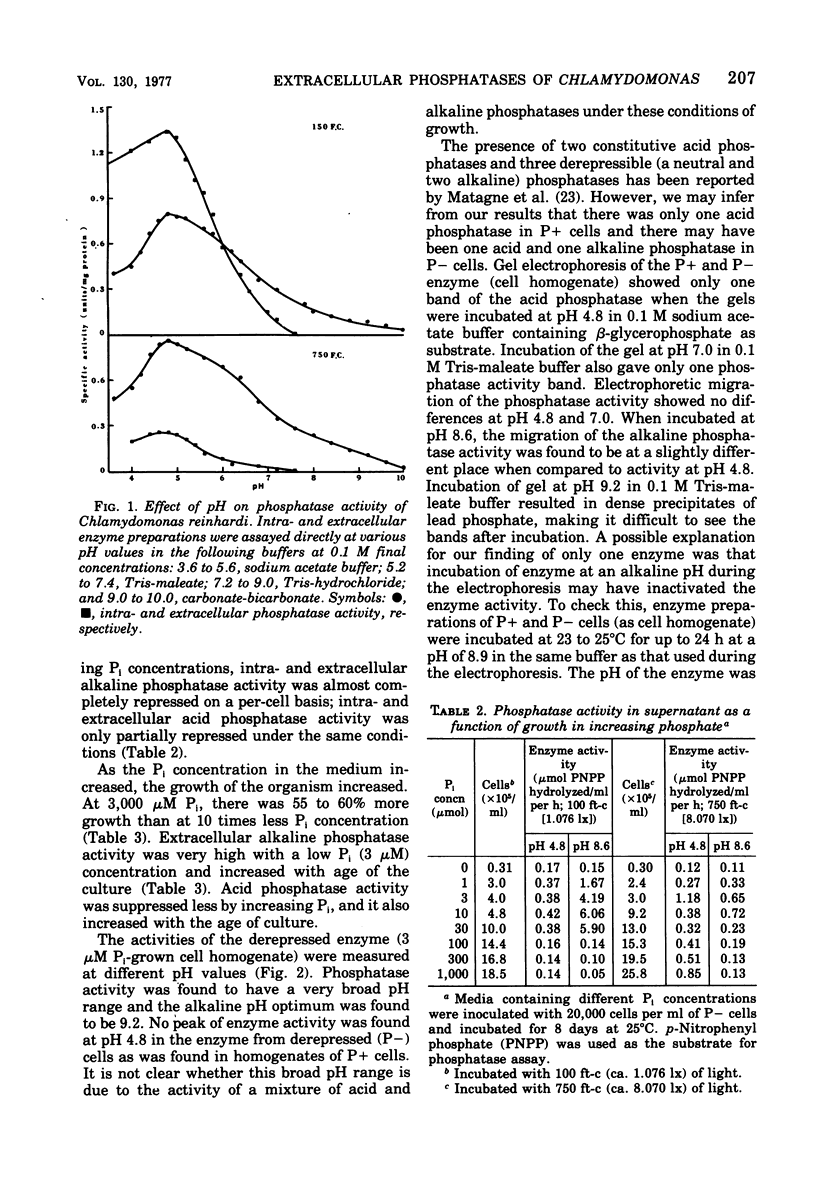
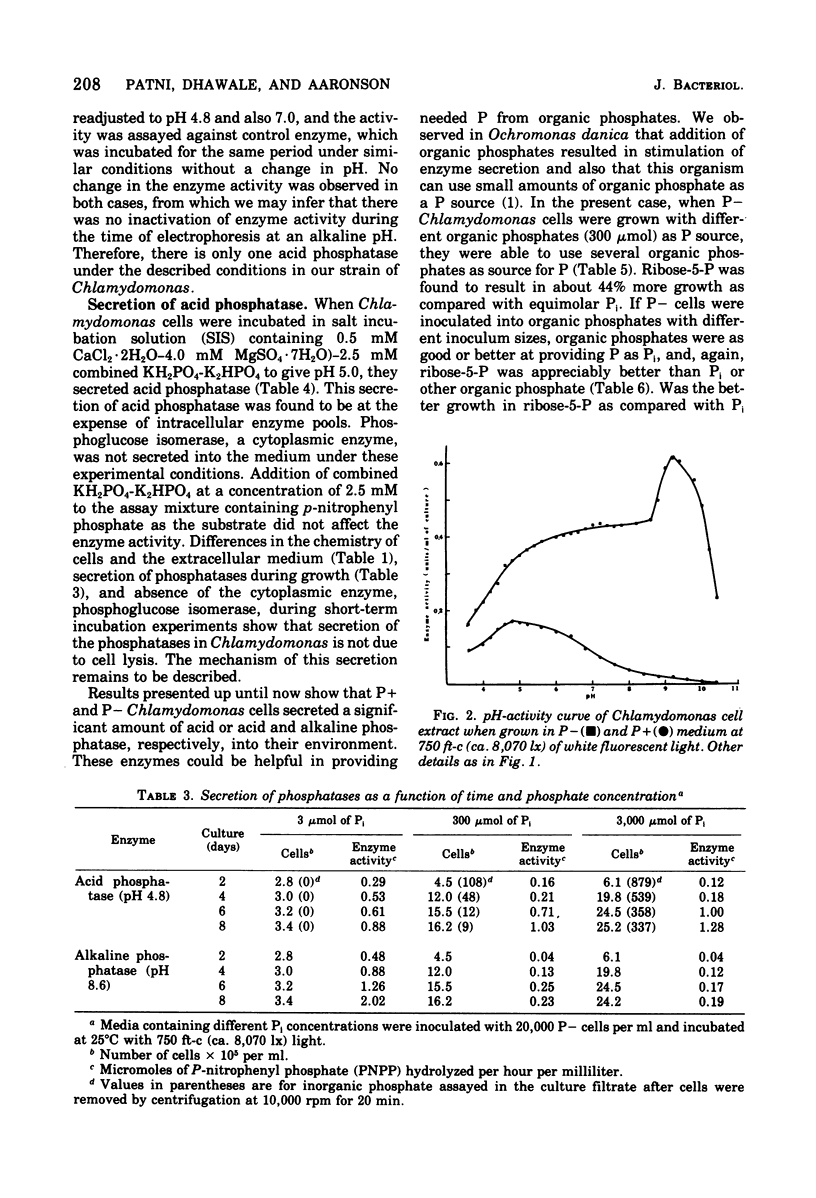
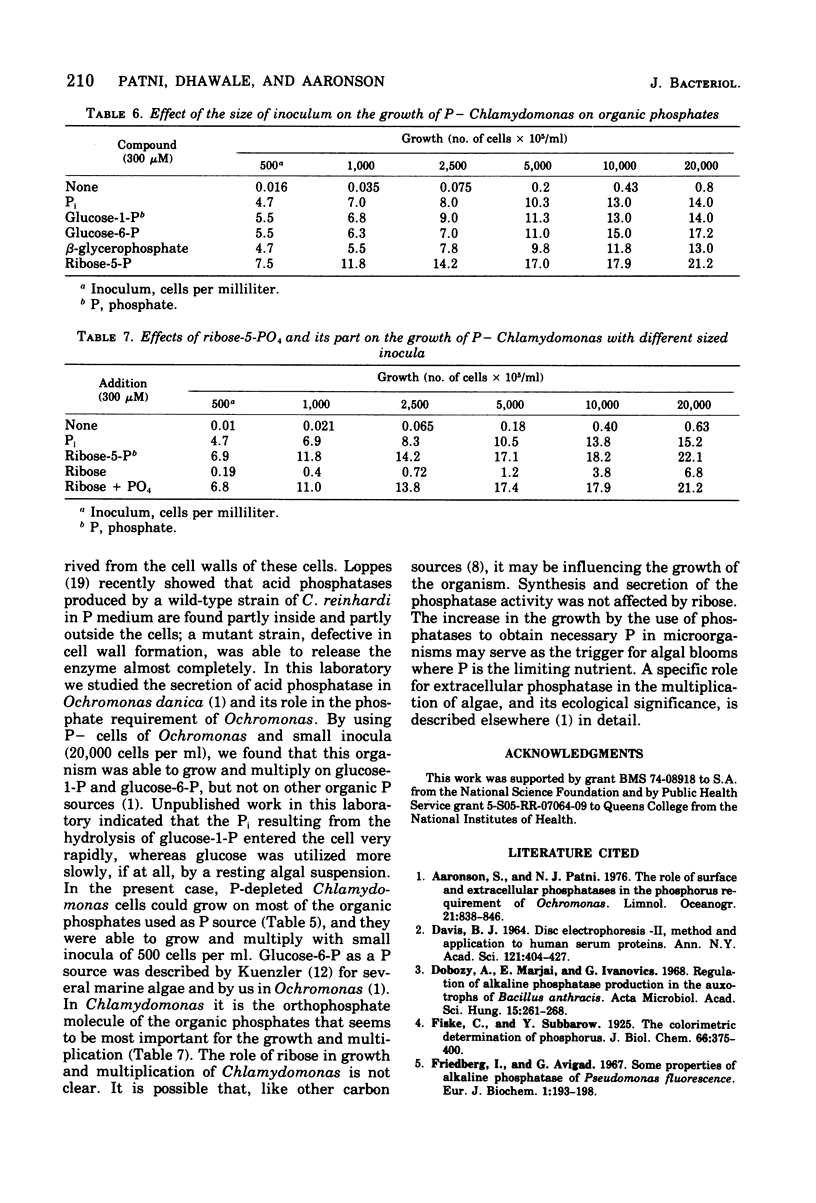
Chlamydomonas reinhardi, cultured under normal growth conditions, secreted significant amounts of protein and carbohydrates but not lipids or nucleic acids. A fivefold increase in light intensity led to a tenfold increase in secreted protein and carbohydrate. Among the proteins secreted was acid phosphatase with a pH optimum at 4.8 like the enzyme in the cells. Phosphorus depleted algae grown on minimal orthophosphate contained and secreted both acid and alkaline phosphatase. The pH optimum of the intracellular alkaline phosphatase was 9.2. When phosphorus-depleted cells were grown with increasing orthophosphate, intra- and extracellular alkaline phosphatase was almost completely repressed and intra- and extracellular acid phosphatase was partially repressed. Extracellular acid and alkaline phosphatase increased with the age of the culture. Electrophoresis indicated only one acid and one alkaline phosphatase in phosphorus-satisfied and phosphorus-depleted cells. Chlamydomonas cells suspended in an inorganic salt solution secreted only acid phosphatase; the absence of any extr-cellular cytoplasmic marker enzyme indicated that there was little, if any, autolysis to account for the extracellular acid enzyme. Phosphorus-depleted cells were able to grow on organic phosphates as the sole source of orthophosphate. Ribose-5-phosphate was the best for cell multiplication, and its utility was shown to be due to the cell's ability to use the ribose as well as the orthophosphatase for cell multiplication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dobozy A., Marjai E., Ivánovics G. Regulation of alkaline phosphatase production in the auxotrophs of Bacillus anthracis. Acta Microbiol Acad Sci Hung. 1968;15(3):261–268. [PubMed] [Google Scholar]
- Friedberg I., Avigad G. Some properties of alkaline phosphatase of Pseudomonas fluorescens. Eur J Biochem. 1967 Apr;1(2):193–198. doi: 10.1007/978-3-662-25813-2_30. [DOI] [PubMed] [Google Scholar]
- GETZ G. S., BARTLEY W. The intracellular distribution of fatty acids in rat liver. The fatty acids of intracellular compartments. Biochem J. 1961 Feb;78:307–312. doi: 10.1042/bj0780307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini A. M., Cremona T., Preddie E. C. Influence of beta-glycerophosphate, acetate and CO2 in the appearance of an alkaline phosphatase in Chlamydomonas reinhardii. Biochem Biophys Res Commun. 1971 Feb 5;42(3):558–563. doi: 10.1016/0006-291x(71)90407-4. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Nyc J. F. Repressible alkaline phosphatase in Neurospora crassa. 3. Enzymatic properties. J Biol Chem. 1969 Oct 10;244(19):5125–5130. [PubMed] [Google Scholar]
- Knutsen G. Repressed and derepressed synthesis of phosphatass during synchronous growth of Chorella pyrenoidosa. Biochim Biophys Acta. 1968 Jun 18;161(1):205–214. doi: 10.1016/0005-2787(68)90310-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liedtke M. P., Ohmann E. Eigenschaften und Regulation einer Phosphatase in Euglena gracilis, Synthese und Inaktivierung. Eur J Biochem. 1969 Oct;10(3):539–548. doi: 10.1111/j.1432-1033.1969.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Lien T., Knutsen G. Synchronous cultures of Chlamydomonas reinhardti. Synthesis of repressed and derepressed phosphatase during the life cycle. Biochim Biophys Acta. 1972 Nov 16;287(1):154–163. [PubMed] [Google Scholar]
- Loppes R., Deltour R. Changes in phosphatase activity associated with cell wall defects in Chlamydomonas reinhardi. Arch Microbiol. 1975 May 5;103(3):247–250. doi: 10.1007/BF00436357. [DOI] [PubMed] [Google Scholar]
- Loppes R., Matagne R. F. Acid phosphatase mutants in Chlamydomonas: isolation and characterization by biochemical, electrophoretic and genetic analysis. Genetics. 1973 Dec;75(4):593–604. doi: 10.1093/genetics/75.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppes R. Release of enzymes by normal and wall-free cells of Chlamydomonas. J Bacteriol. 1976 Oct;128(1):114–116. doi: 10.1128/jb.128.1.114-116.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne R. F., Loppes R., Deltour R. Phosphatase of Chlamydomonas reinhardi: biochemical and cytochemical approach with specific mutants. J Bacteriol. 1976 May;126(2):937–950. doi: 10.1128/jb.126.2.937-950.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses V. The regulatory process in the de-repression of enzyme synthesis. Alkaline phosphatase of Bacillus subtilis. Biochem J. 1967 Jun;103(3):650–659. doi: 10.1042/bj1030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Secretion of acid hydrolases and its intracellular source in Tetrahymena pyriformis. J Cell Biol. 1972 Feb;52(2):478–487. doi: 10.1083/jcb.52.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni N. J., Aaronson S., Holik K. J., Davis R. H. Existence of acid and alkaline phosphohydrolase activity in the phytoflagellate Ochromonas danica. Arch Mikrobiol. 1974 Apr 10;97(1):63–67. doi: 10.1007/BF00403045. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Gibbs M. Partial purification and characterization of two fructose diphosphate aldolases from Chlamydomonas mundana. Biochim Biophys Acta. 1967 Jan 11;132(1):145–154. doi: 10.1016/0005-2744(67)90200-8. [DOI] [PubMed] [Google Scholar]
- Schurr A., Yagil E. Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol. 1971 Mar;65(3):291–303. doi: 10.1099/00221287-65-3-291. [DOI] [PubMed] [Google Scholar]
- Shah D. B., Blobel H. Repressible alkaline phosphatase of Staphylococcus aureus. J Bacteriol. 1967 Sep;94(3):780–781. doi: 10.1128/jb.94.3.780-781.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]


