Abstract
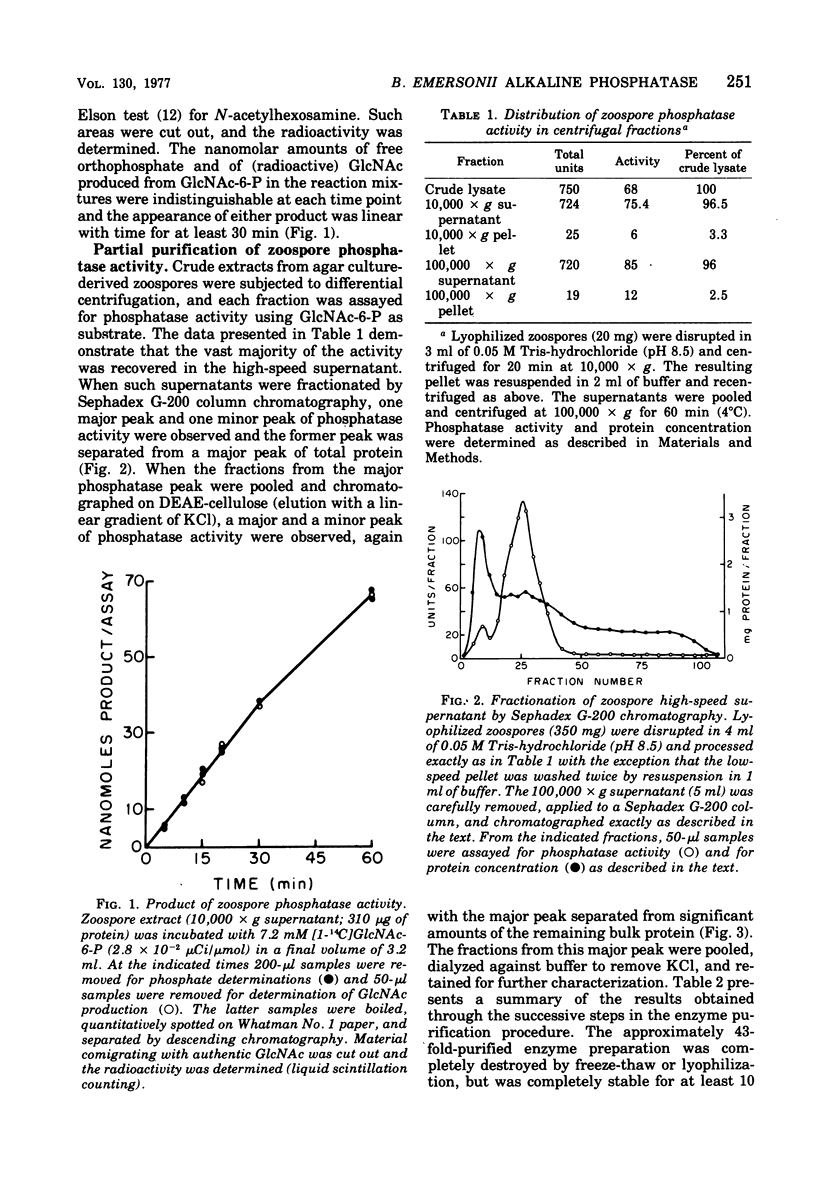
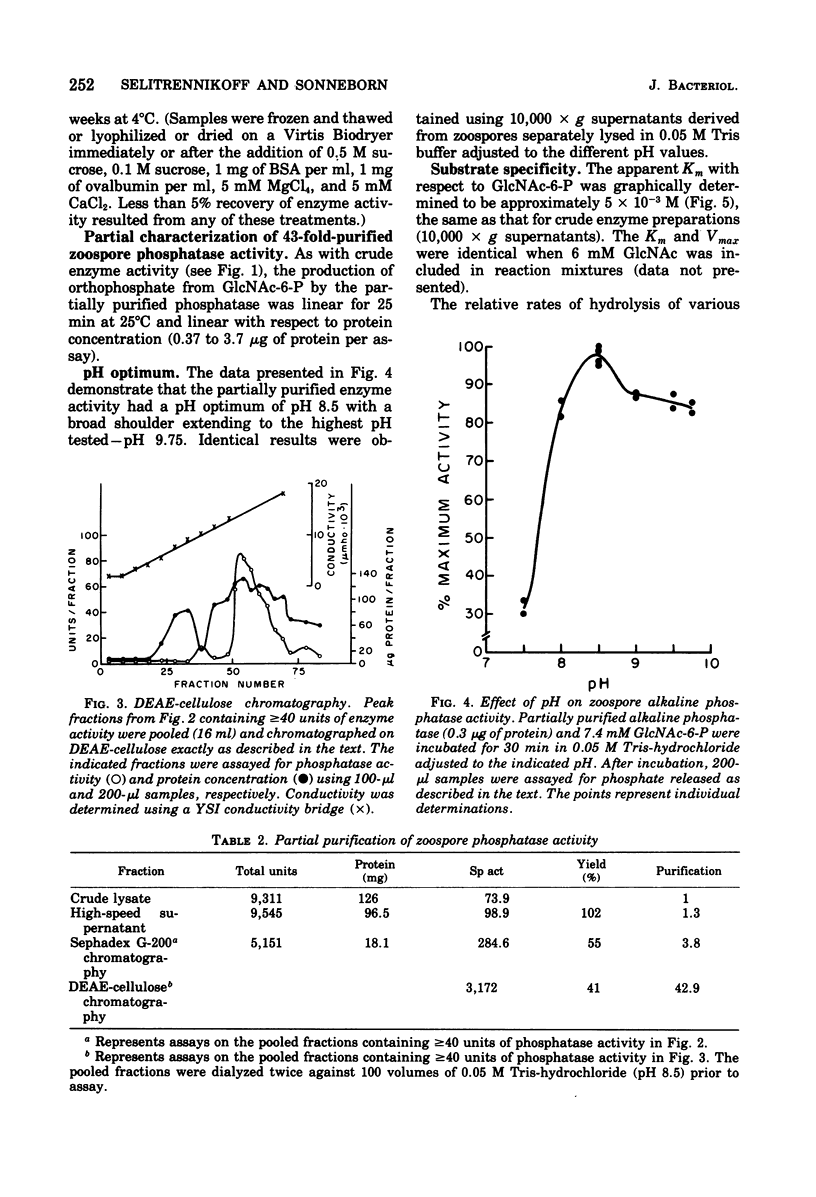
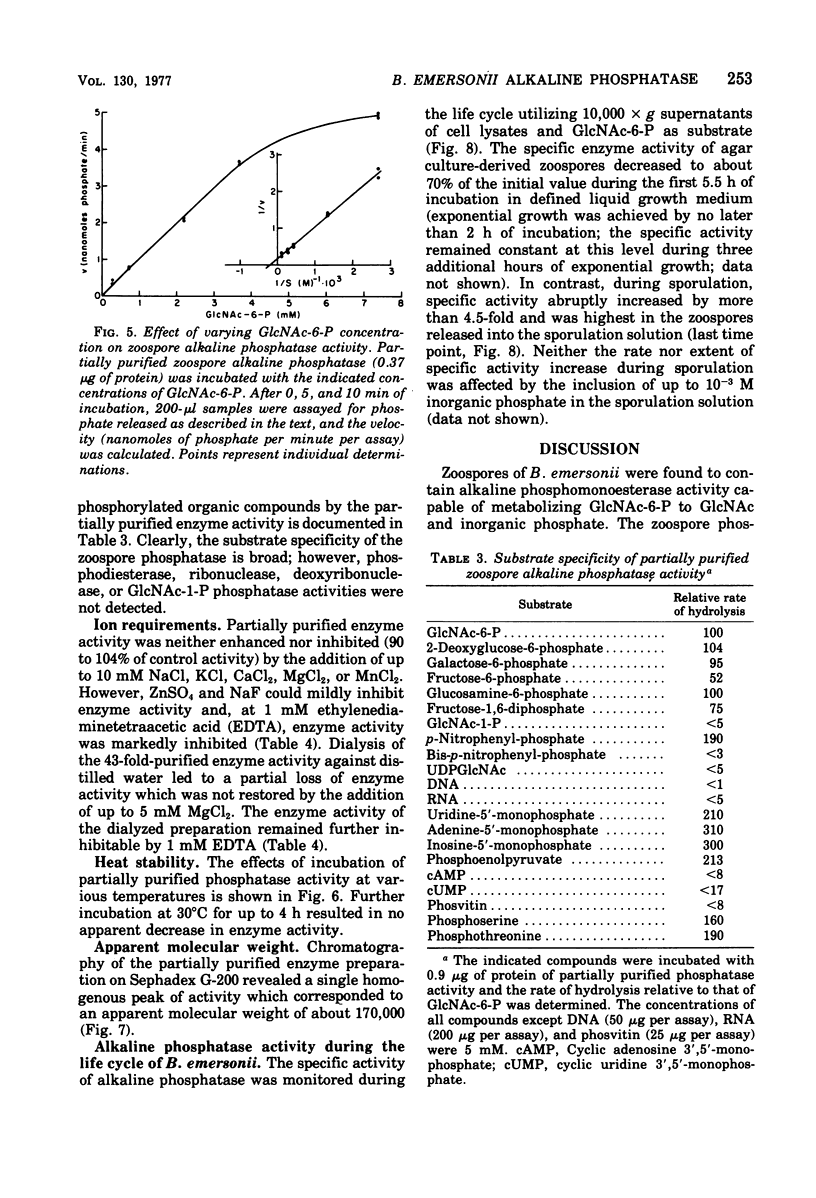
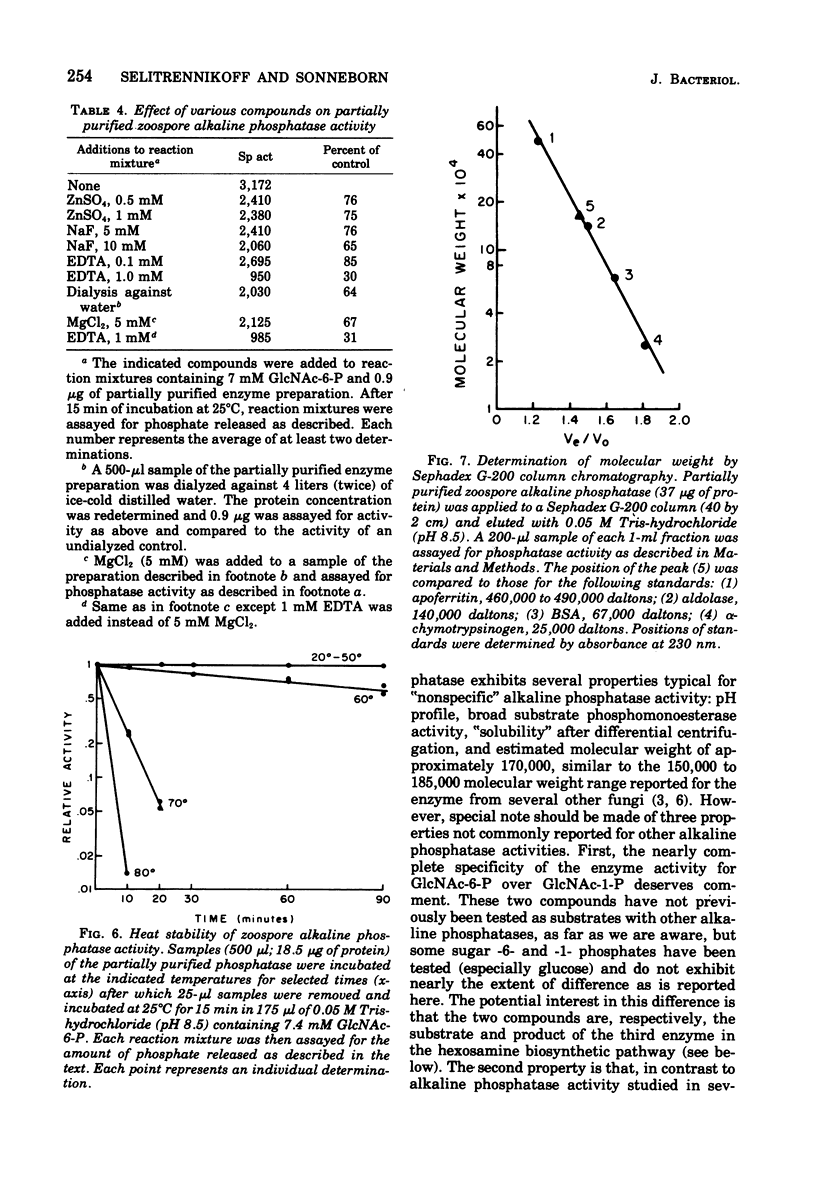
Alkaline phosphomonoesterase (EC 3.1.3.1) activity from Blastocladiella emersonii, while displaying typically broad substrate specificity for phosphorylated organic compounds, exhibited nearly complete substrate preference for N-acetylglucosamine-6-phosphate over N-acetylglucosamine-1-phosphate. Enzyme in zoospore extracts was purified 43-fold by differential centrifugation followed by gel filtration (Sephadex G-200) and then by ion-exchange chromatography (diethylaminoethyl-cellulose). The partially purified enzyme displayed an apparent molecular weight (Sephadex G-200) of approximately 170,000. The activity of partially purified enzyme exhibited a pH optimum of pH 8.5, did not require a metal divalent cation, but was inhibitable by ethylenediaminetetraacetic acid. During the life cycle of the organism, the specific activity of the phosphatase decreased slightly during germination and early exponential growth but then increased about 4.5-fold during sporulation. B. emersonii alkaline phosphatase does not appear to be a repressible enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camargo E. P., Dietrich C. P., Sonneborn D., Strominger J. L. Biosynthesis of chitin in spores and growing cells of Blastocladiella emersonii. J Biol Chem. 1967 Jul 10;242(13):3121–3128. [PubMed] [Google Scholar]
- Dorn G. L. Purification and characterization of phosphatase I from Aspergillus nidulans. J Biol Chem. 1968 Jun 25;243(12):3500–3506. [PubMed] [Google Scholar]
- Dorn G., Rivera W. Kinetics of fungal growth and phosphatase formation in Aspergillus nidulans. J Bacteriol. 1966 Dec;92(6):1618–1622. doi: 10.1128/jb.92.6.1618-1622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Toh-E A., Uno I., Hasunuma K. Isolation and characterization of nuclease mutants in Neurospora crassa. Genetics. 1969 Sep;63(1):75–92. doi: 10.1093/genetics/63.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950 Mar;33(4):349–362. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Nyc J. F., Brown D. M. A repressible alkaline phosphatase in Neurospora crassa. II. Isolation and chemical properties. J Biol Chem. 1968 Jun 10;243(11):3076–3082. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nyc J. F., Kadner R. J., Crocken B. J. A repressible alkaline phosphatase in Neurospora crassa. J Biol Chem. 1966 Apr 10;241(7):1468–1472. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- Selitrennikoff C. P., Allin D., Sonneborn D. R. Chitin biosynthesis during Blastocladiella zoospore germination: evidence that the hexosamine biosynthetic pathway is post-translationally activated during cell differentiation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):534–538. doi: 10.1073/pnas.73.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Bromberg R., Sonneborn D. R. Zoospore germination in the water mold. Blastocladiella emersonii. I. Measurement of germination and sequence of subcellular morphological changes. Dev Biol. 1969 Sep;20(3):183–217. doi: 10.1016/0012-1606(69)90012-8. [DOI] [PubMed] [Google Scholar]



