Abstract
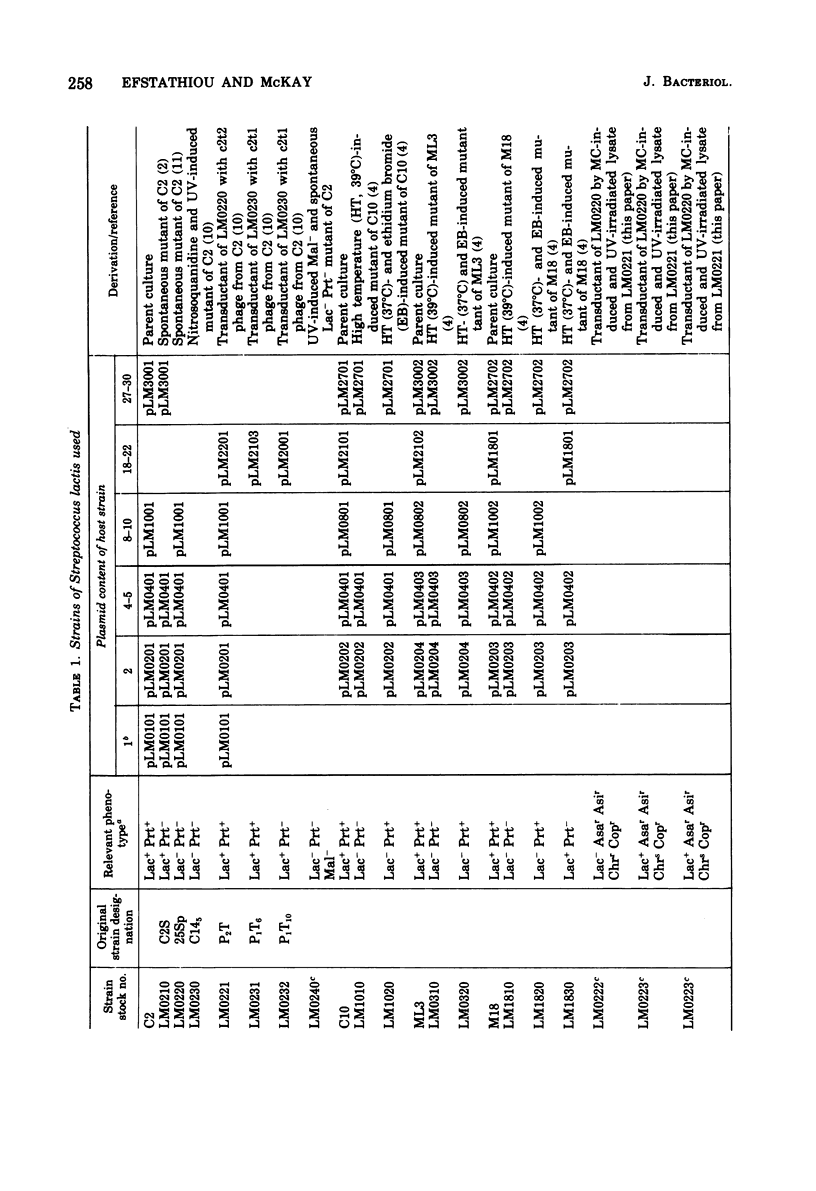
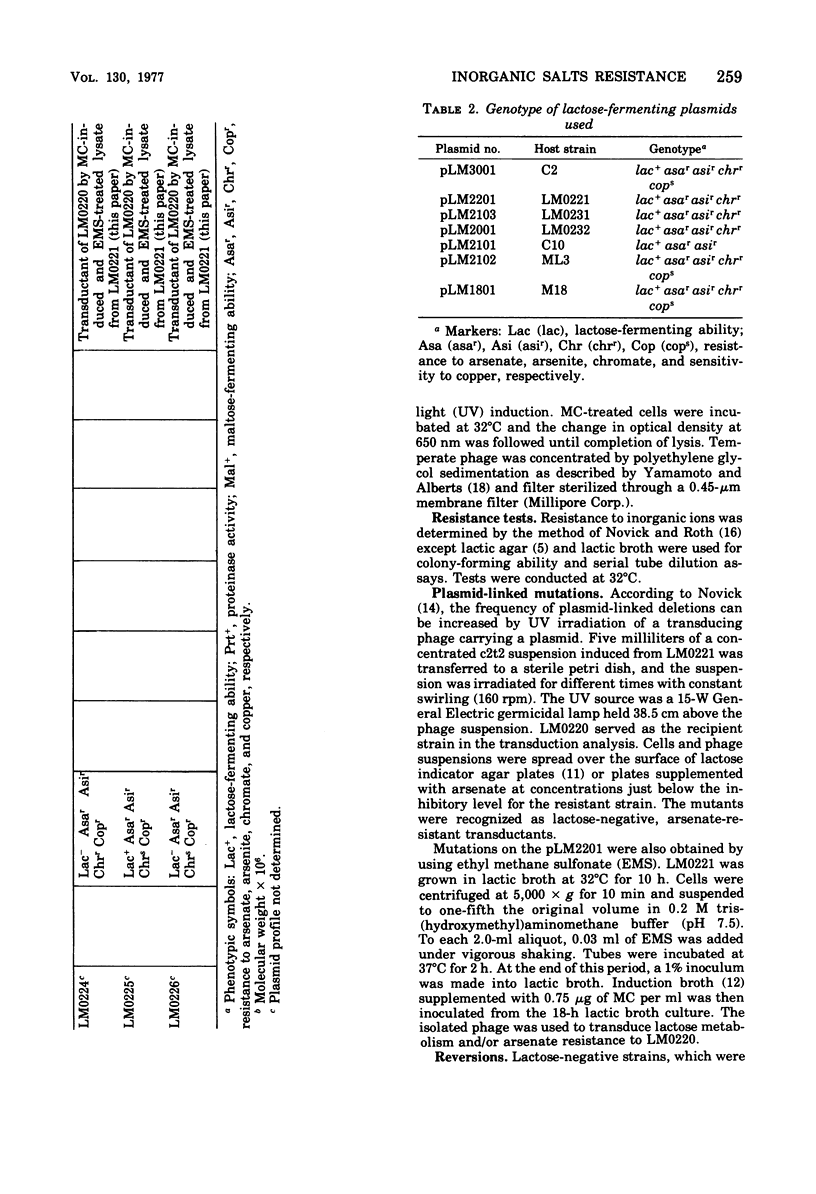
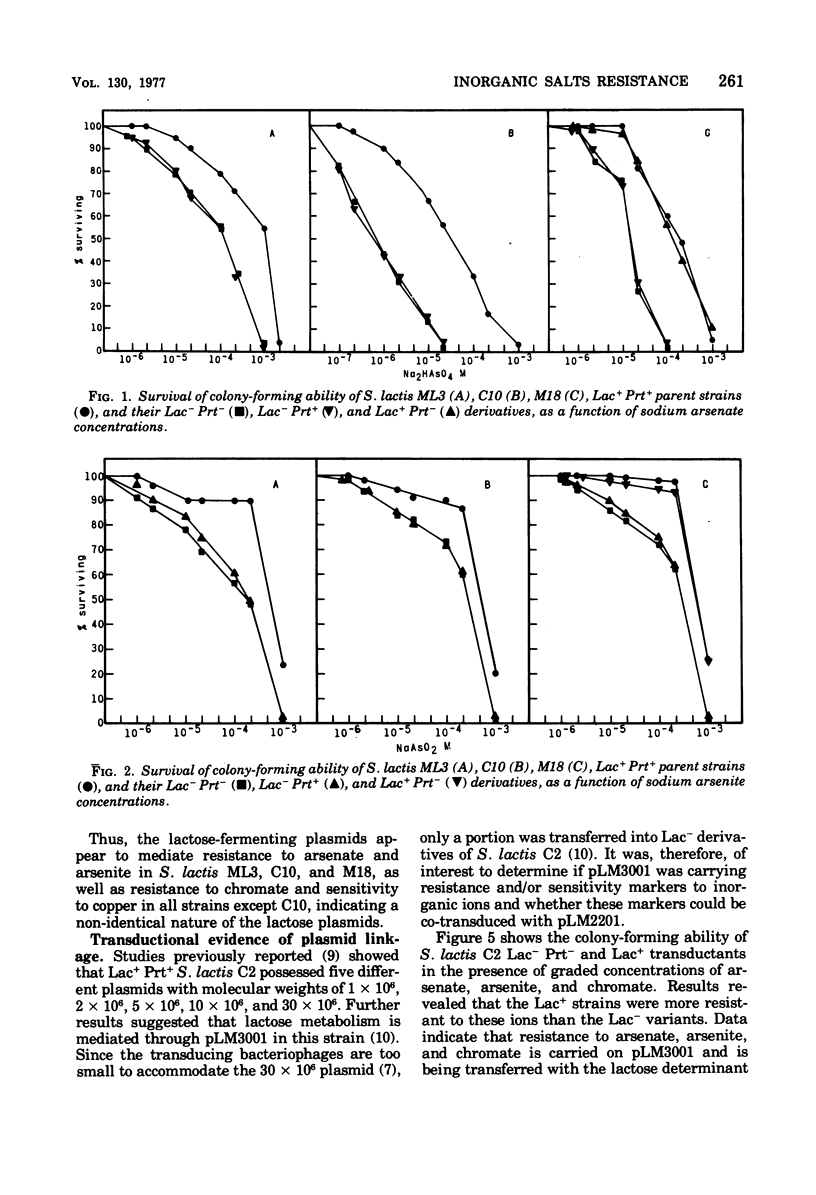
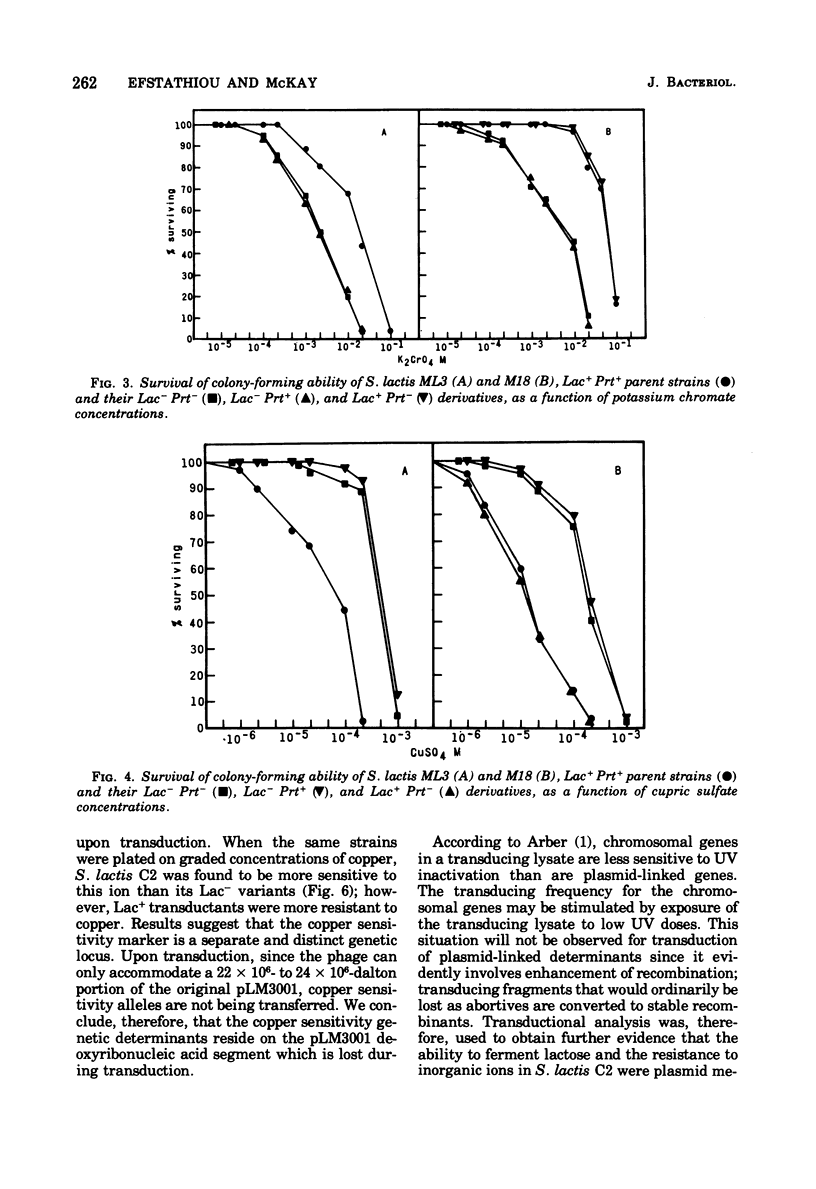
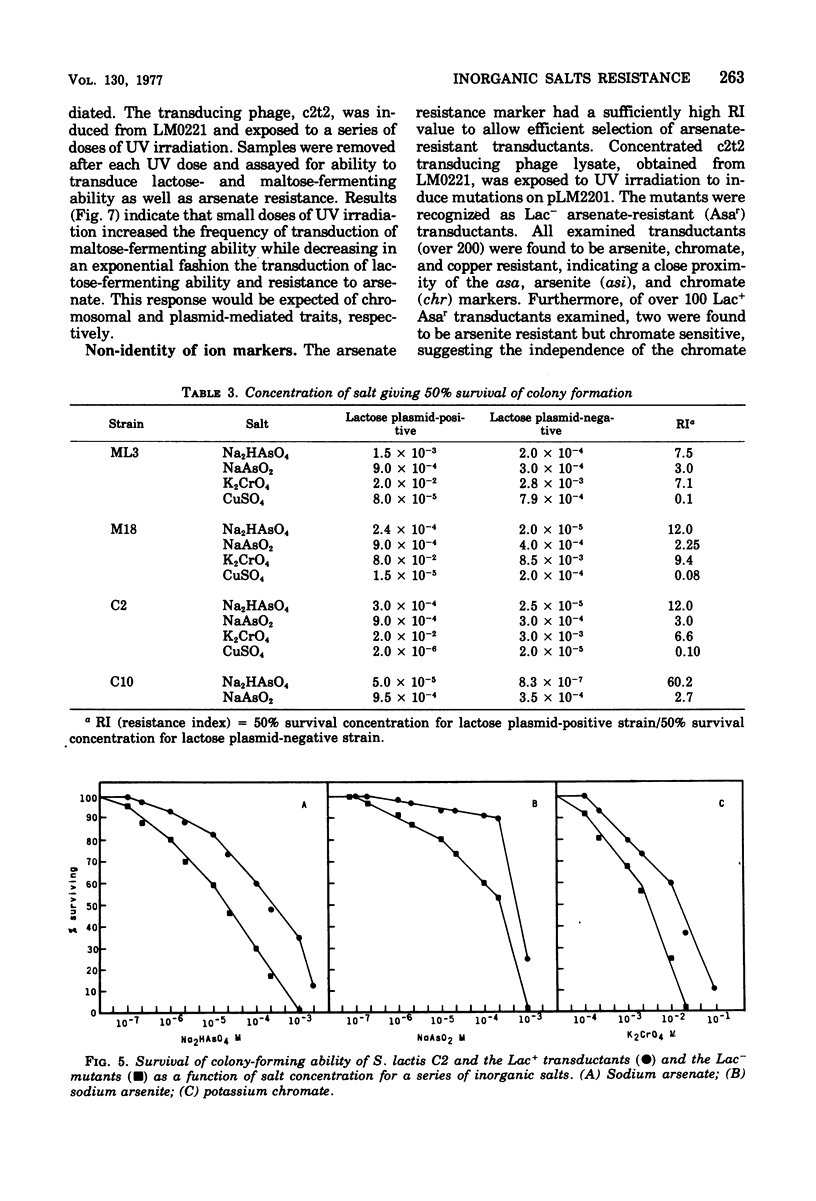
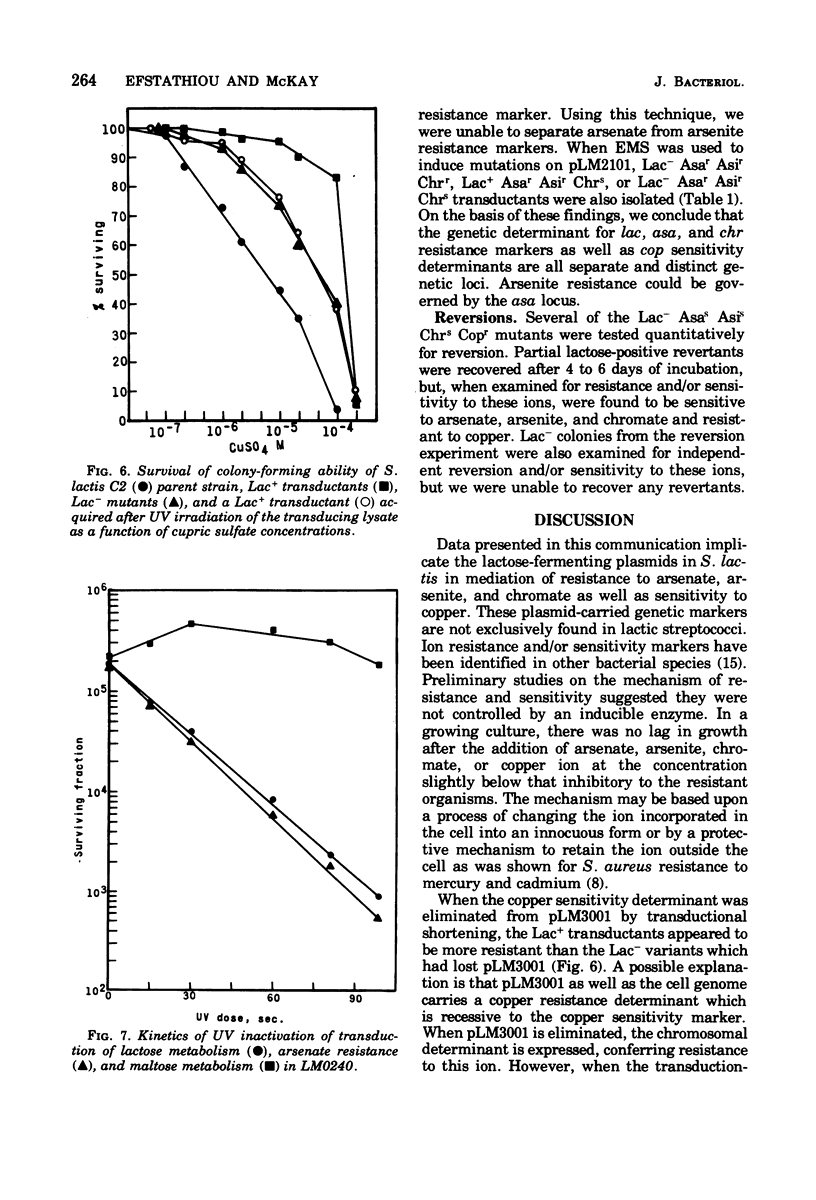
Present evidence indicates that lactose metabolism in group N streptococci is linked to plasmid deoxyribonucleic acid. Lactose-positive (Lac+) Streptococcus lactis and lactose-negative (Lac-) derivatives were examined for their resistance to various inorganic ions. Lac+ S. lactis strains ML3, M18, and C2 were found more resistant to arsenate (7.5- to 60.2-fold), arsenite (2.25- to 3.0-fold), and chromate (6.6- to 9.4-fold), but more sensitive to copper (10.0- to 13.3-fold) than their Lac- derivatives. These results suggested that genetic information for resistance and/or sensitivity to these ions resides on the "lactose plasmid." Kinetics of ultraviolet irradiation inactivation of transducing ability for lactose metabolism and arsenate resistance confirmed the plasmid location of the two markers. Lac+ transductants from S. lactis C2 received genetic determinants for resistance to arsenate, arsenite, and chromate but not for copper sensitivity. In this case, resistance markers were lost when the transductants became Lac- but the derivatives remained copper resistant. The resistant markers for arsenate and arsenite could not be identified as separate genetic loci, but chromate resistance and copper sensitivity markers were found to be independent genetic loci. The "lactose plasmid" from S. lactis C10 possessed the genetic loci for arsenate and arsenite resistance but not for chromate resistance or copper sensitivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- CITTI J. E., SANDINE W. E., ELLIKER P. R. COMPARISON OF SLOW AND FAST ACID-PRODUCING STREPTOCOCCUS LACTIS. J Dairy Sci. 1965 Jan;48:14–18. doi: 10.3168/jds.s0022-0302(65)88152-8. [DOI] [PubMed] [Google Scholar]
- Danbara H., Yoshikawa M. Plasmid-determined epistatic susceptibility to kasugamycin. Antimicrob Agents Chemother. 1975 Sep;8(3):243–250. doi: 10.1128/aac.8.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Plasmids in Streptococcus lactis: evidence that lactose metabolism and proteinase activity are plasmid linked. Appl Environ Microbiol. 1976 Jul;32(1):38–44. doi: 10.1128/aem.32.1.38-44.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L. Isolation and examination of transducing bacteriophage particles from Streptococcus lactis C2. J Dairy Sci. 1976 Mar;59(3):396–404. doi: 10.3168/jds.s0022-0302(76)84219-1. [DOI] [PubMed] [Google Scholar]
- Kondo I., Ishikawa T., Nakahara H. Mercury and cadmium resistances mediated by the penicillinase plasmid in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):1–7. doi: 10.1128/jb.117.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Plasmid distribution and evidence for a proteinase plasmid in Streptococcus lactis C2-1. Appl Microbiol. 1975 Apr;29(4):546–548. doi: 10.1128/am.29.4.546-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Novick R. P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972 Nov;112(2):761–772. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



