Abstract
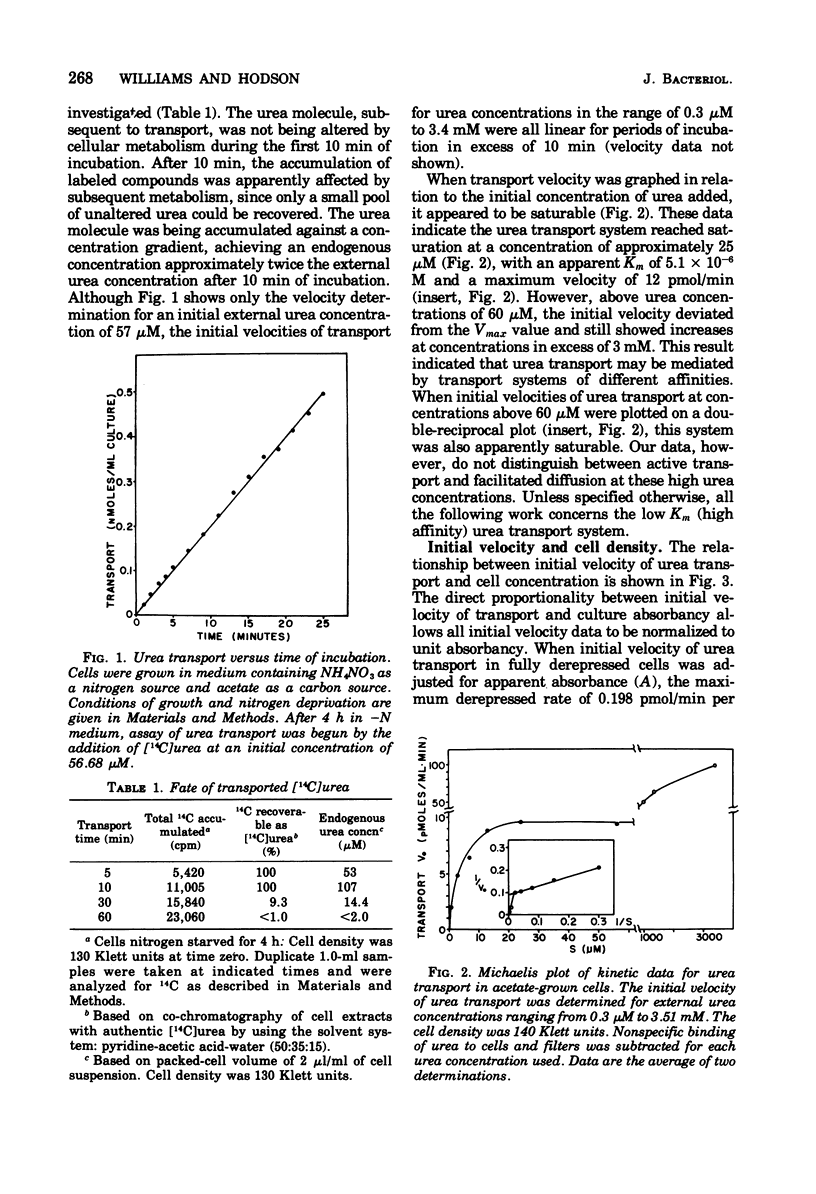
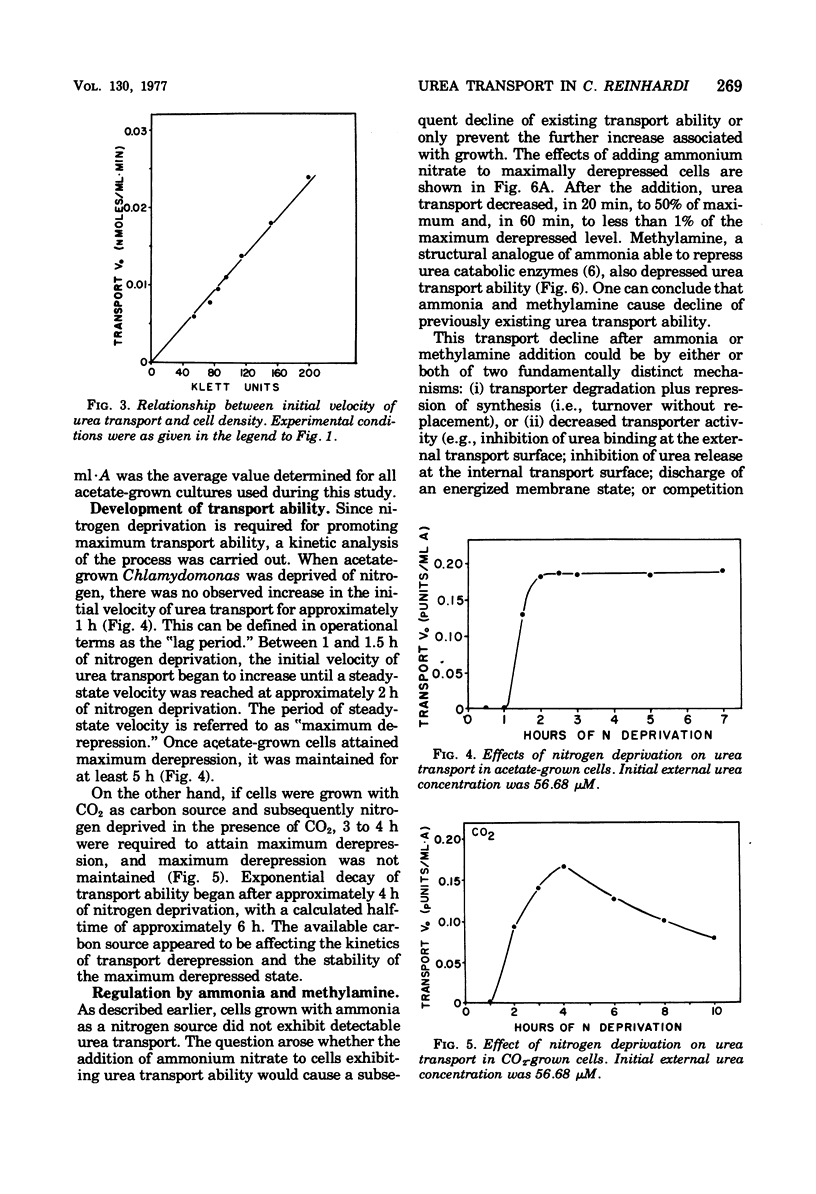
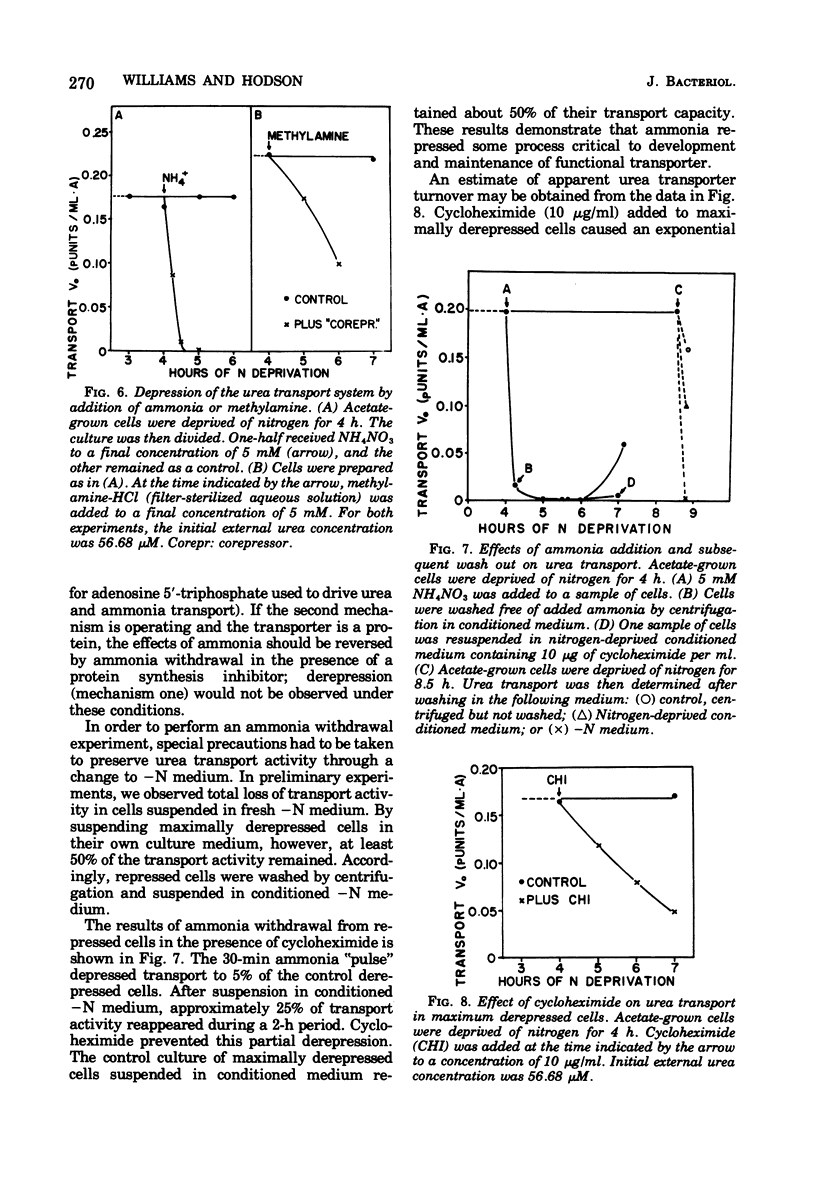
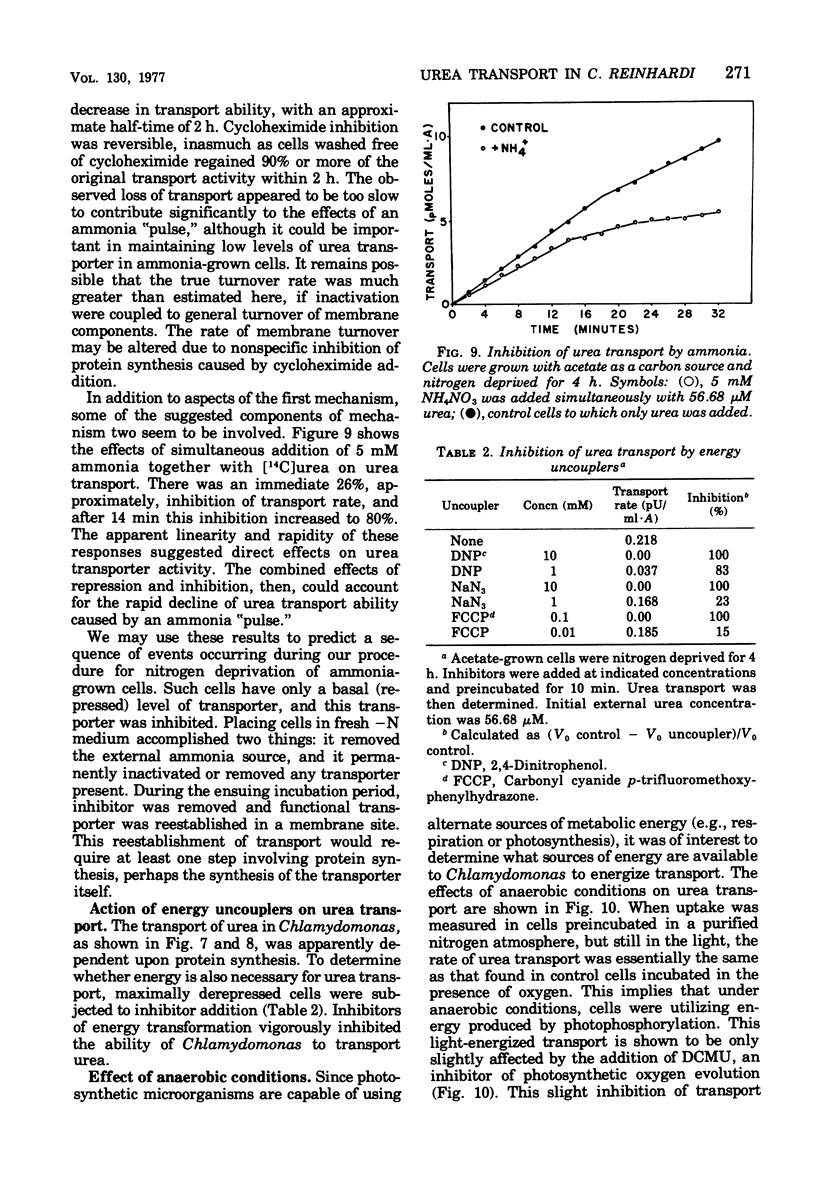
Urea transport into the unicellular green alga Chlamydomonas reinhardi was investigated to further our understanding of controls operating on urea catabolism in this organism. Transport into cells grown with acetate and deprived of ammonia is a saturable process, mediated by at least two systems operating maximally at different external urea concentrations. The lower concentration system, with an apparent Km for urea of 5.1 micron, was the object of detailed study. Transport of urea from a saturating concentration (57 micron) into ammonia- and acetate-grown cells freshly suspended in ammonia-limited medium was not detected. Upon further culturing in the absence of ammonia, derepression occurred with transport ability, first appearing at about 1 h , reaching a maximum at about 2 h, and maintaining this maximum at least 5 h. In contrast to this, CO2-grown cells became derepressed more slowly, and maximum transport ability was not maintained. Addition of ammonia or methylamine (5 mM) during nitrogen deprivation prevented further increases in transport ability and caused loss of previously acquired transport ability. Cycloheximide (10 microng/ml) had a similar effect. Energy uncouplers or dark, anaerobic conditions depressed transport. By these criteria, transport from low urea concentrations is mediated by a process that requires protein synthesis and activation by cellular energy, and the process has a rapid rate of turnover and of deactivation by ammonia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr, Cove D. J. Methylammonium resistance in Aspergillus nidulans. J Bacteriol. 1969 Jun;98(3):1284–1293. doi: 10.1128/jb.98.3.1284-1293.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Sumrada R. Urea transport in Saccharomyces cerevisiae. J Bacteriol. 1975 Feb;121(2):571–576. doi: 10.1128/jb.121.2.571-576.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera J., Paneque A., Maldonado J. M., Barea J. L., Losada M. Regulation by ammonia of nitrate reductase synthesis and activity in Chlamydomonas reinhardi. Biochem Biophys Res Commun. 1972 Aug 21;48(4):996–1003. doi: 10.1016/0006-291x(72)90707-3. [DOI] [PubMed] [Google Scholar]
- Hodson R. C., Williams S. K., 2nd, Davidson W. R., Jr Metabolic control of urea catabolism in Chlamydomonas reinhardi and Chlorella pyrenoidosa. J Bacteriol. 1975 Mar;121(3):1022–1035. doi: 10.1128/jb.121.3.1022-1035.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Loos E., Tanner W. A confirmation of the proposed model for the hexose uptake system of Chlorella vulgaris. Anaerobic studies in the light and in the dark. J Membr Biol. 1973;12(1):89–99. doi: 10.1007/BF01869993. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Goodenough U. W. Gametic differentiation in Chlamydomonas reinhardtii. I. Production of gametes and their fine structure. J Cell Biol. 1975 Dec;67(3):587–605. doi: 10.1083/jcb.67.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional control of sexuality in Chlamydomonas reinhardi. J Gen Physiol. 1954 Jul 20;37(6):729–742. doi: 10.1085/jgp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hodson R. C., Williams SK I. I., Howell S. H. The induction of allophanate lyase during the vegetative cell cycle in light-synchronized cultures of Chlamydomonas reinhardi. Biochim Biophys Acta. 1975 Jul 14;399(1):71–78. doi: 10.1016/0304-4165(75)90212-3. [DOI] [PubMed] [Google Scholar]
- Strijkert P. J., Loppes R., Sussenbach J. S. Arginine metabolism in Chlamydomonas reinhardi. Regulation of uptake and breakdown. FEBS Lett. 1971 May 20;14(5):329–332. doi: 10.1016/0014-5793(71)80293-4. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. Urea carboxylase from Saccharomyces cerevisiae. Evidence for a minimal two-step reaction sequence. J Biol Chem. 1973 Jan 10;248(1):325–330. [PubMed] [Google Scholar]



