Abstract
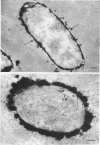
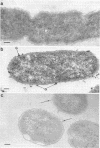
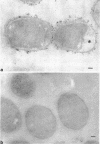
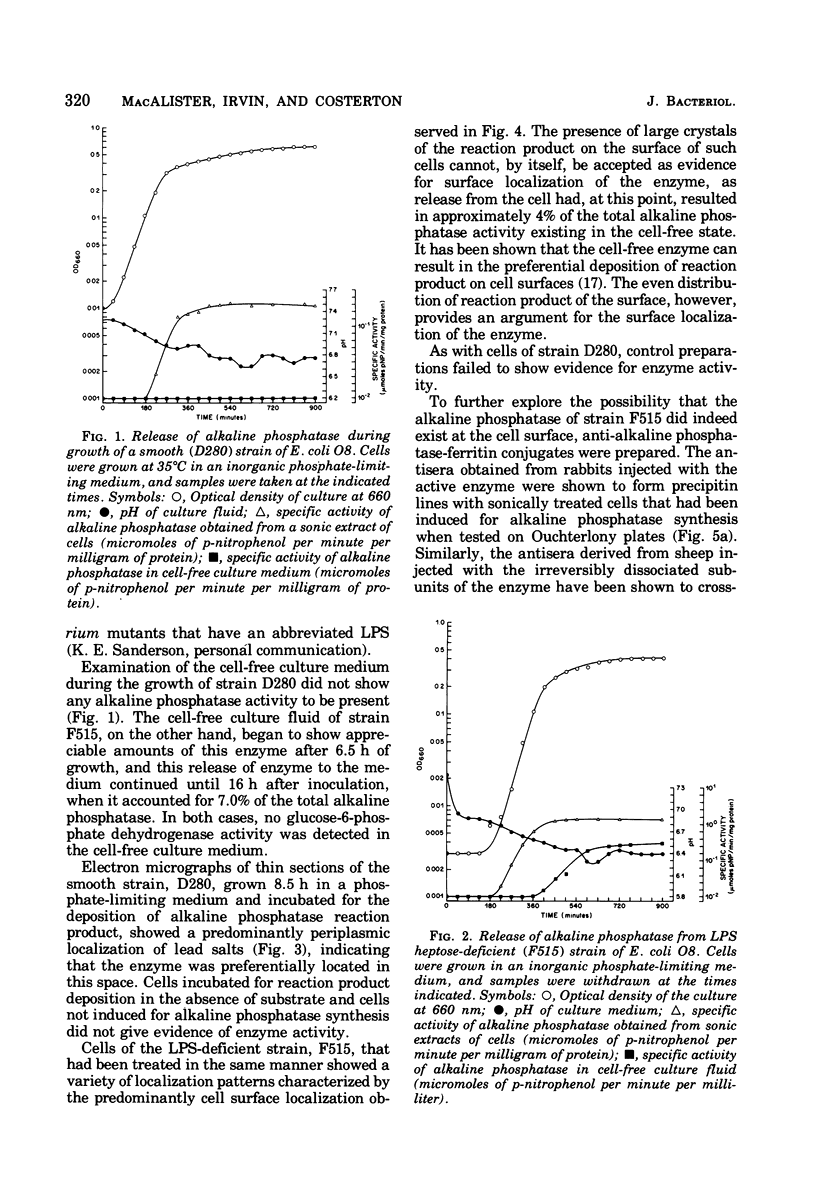
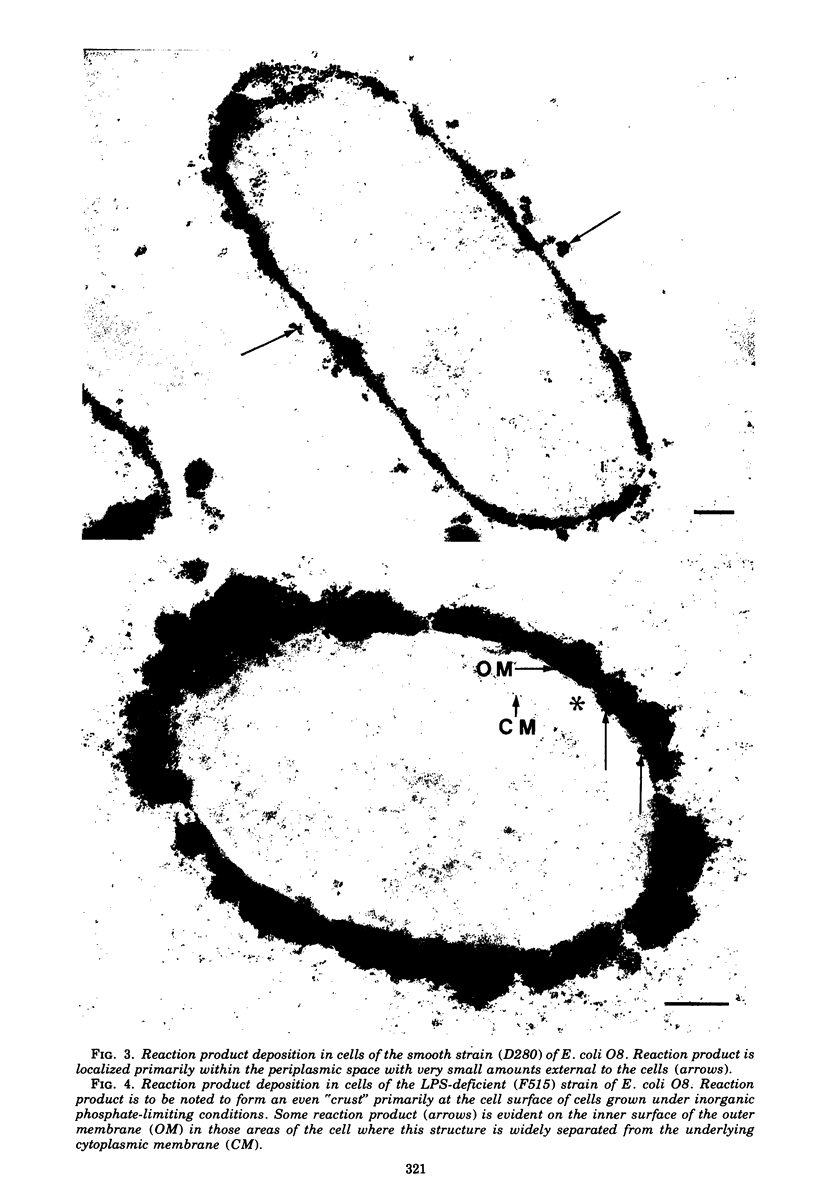
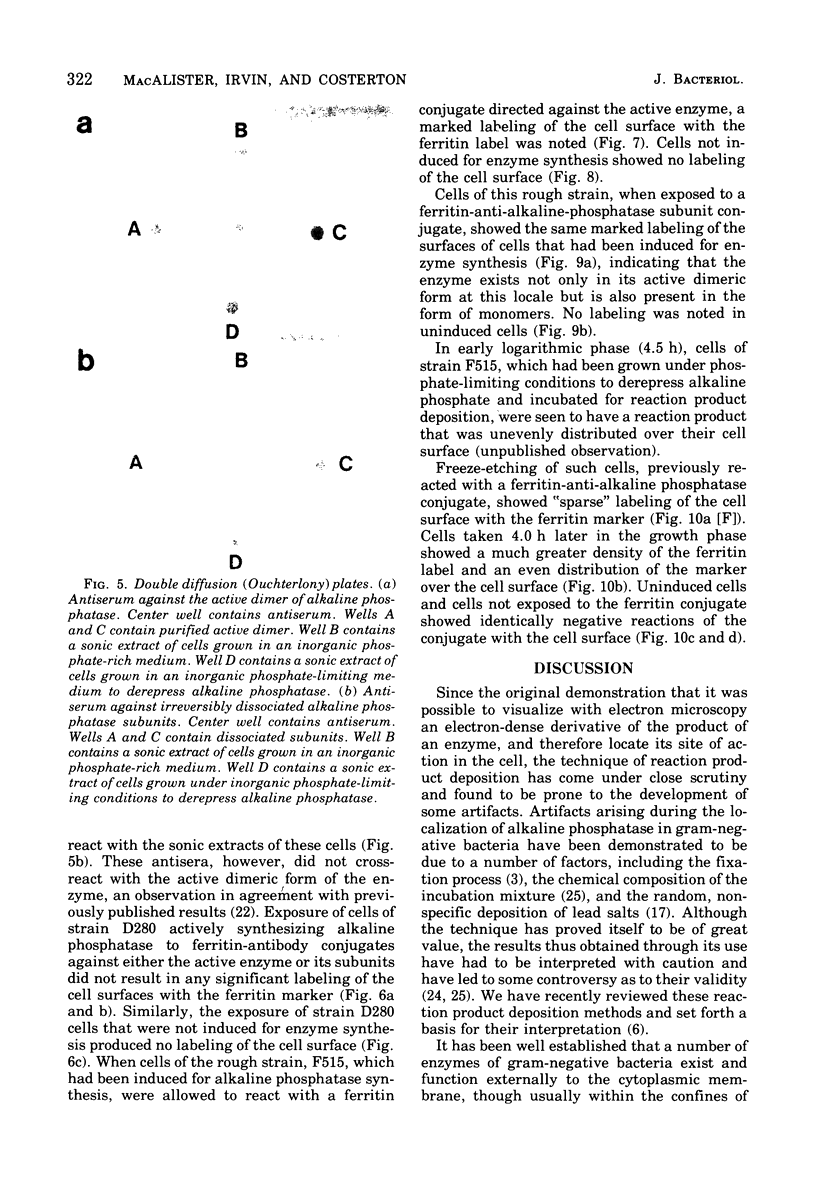
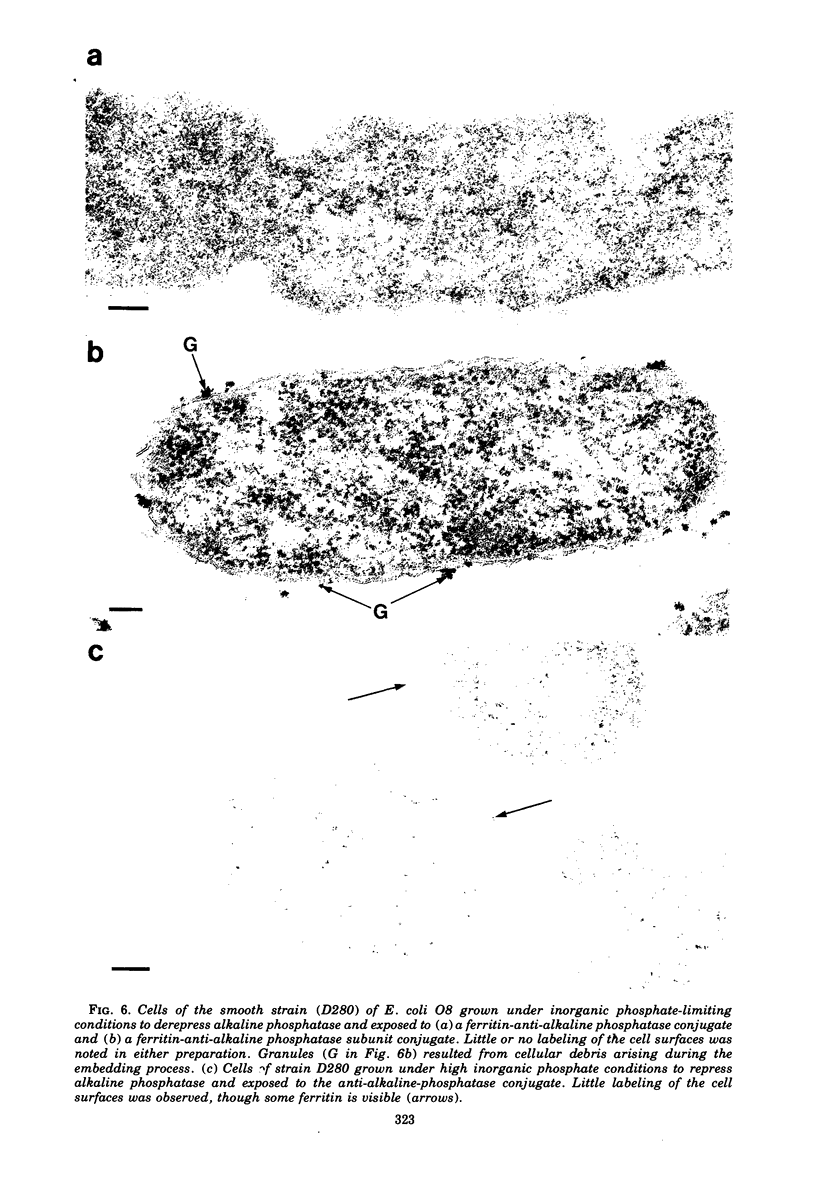
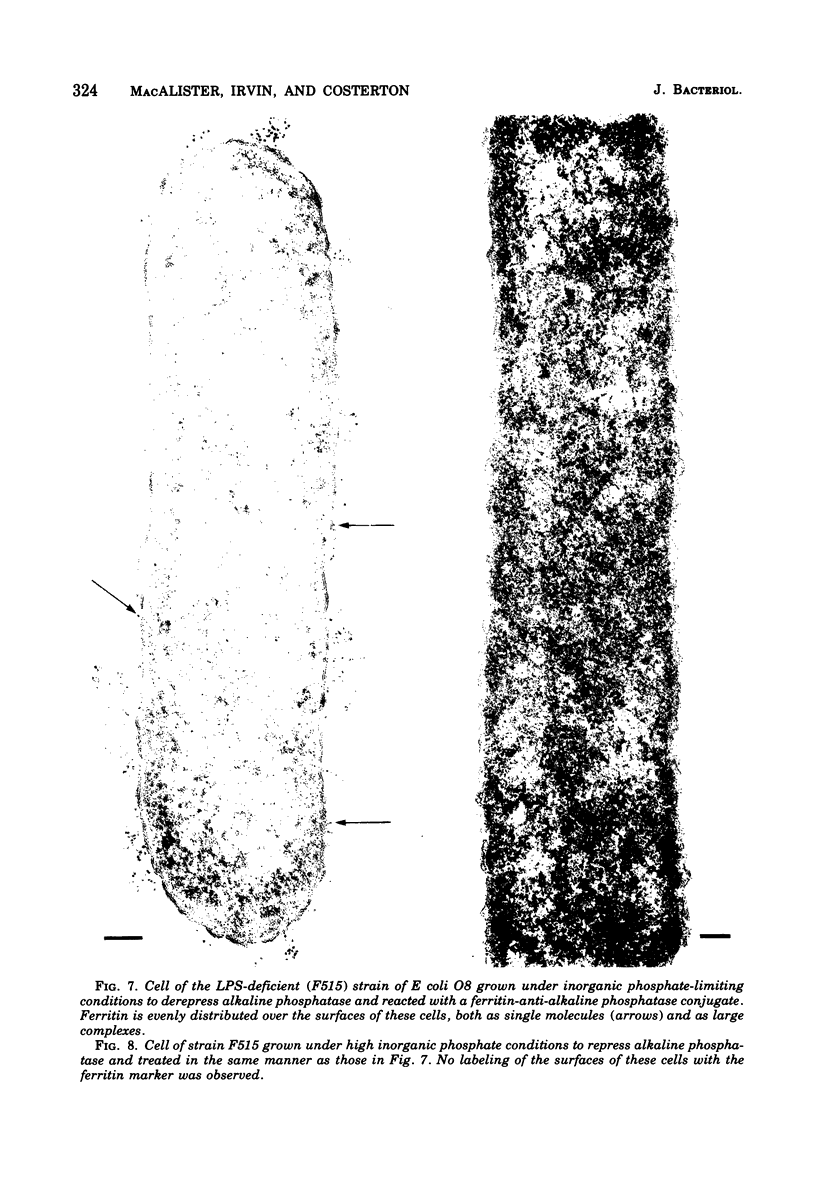
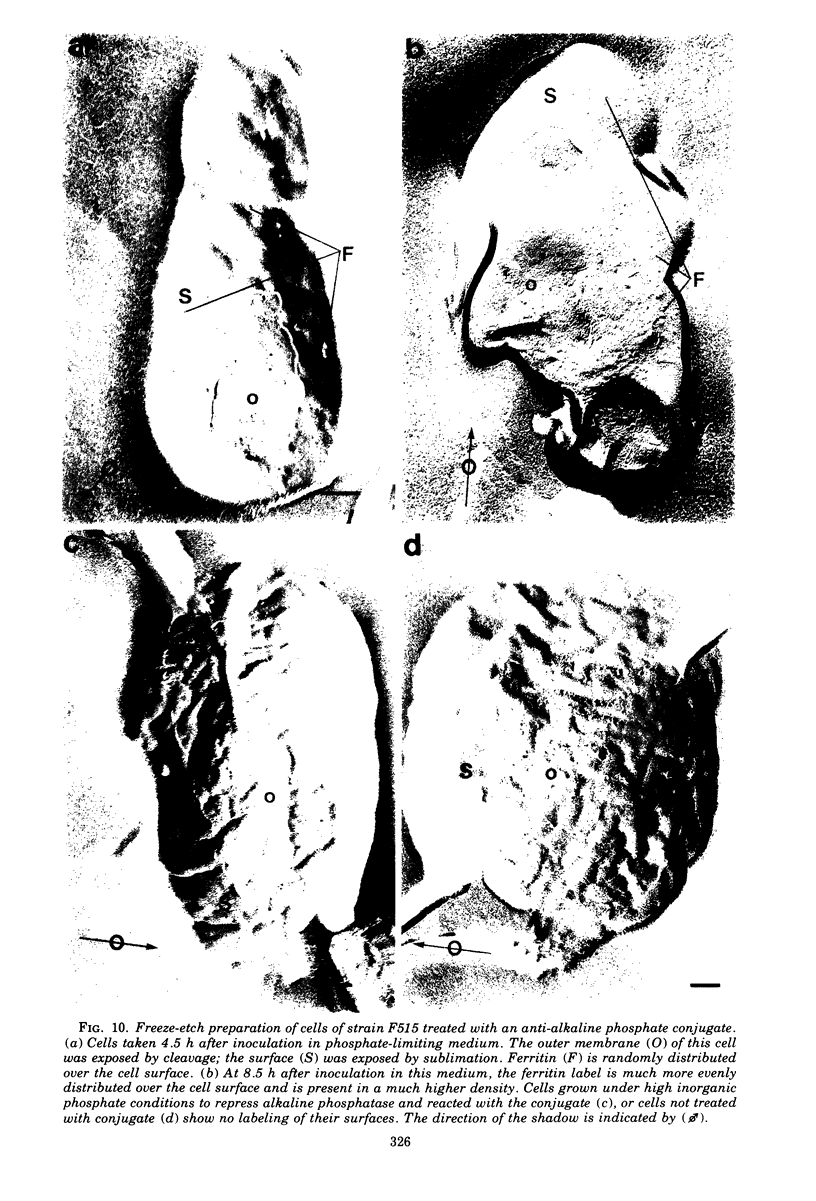
When cells of a wild-type Eschericia coli O8 strain bearing a complete lipopolysaccharide were incubated for alkaline phosphatase reaction product and examined by electron microscopy, the depostion of lead salts was to be observed primarily within the periplasmic space. A similar treatment of cells derived from this strain, which bears a highly abbreviated lipopolysaccharide, showed a mixed cell surface and periplasmic localization of reaction product, suggesting a surface association of a portion of the enzyme. To further explore this possibility, ferritin-antibody conjugates against the active enzyme and its irreversibly dissociated subunits were prepared and allowed to react with cells of both strains. The results obtained from these experiments revealed the presence of both the active enzyme and inactive subunits of the enzyme at the cell surface of the mutant strain. The evidence obtained offers further proof of the validity of the reaction product deposition technique and indicates that alkaline phosphatase may be associated with some component of the outer membrane in this organism. The observation of enzyme subunits at the cell surface further suggests that an association of these subunits with structural components of the cell envelope may provide a locus at which they may dimerize to form active enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beacham I. R., Kahana R., Levy L., Yagil E. Mutants of Escherichia coli K-12 "cryptic," or deficient in 5'-nucleotidase (uridine diphosphate-sugar hydrolase) and 3'-nucleotidase (cyclic phosphodiesterase) activity. J Bacteriol. 1973 Nov;116(2):957–964. doi: 10.1128/jb.116.2.957-964.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971 Jul;107(1):325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csopak H., Garellick G., Hallberg B. Purification of Escherichia coli alkaline phosphatase. Improved growth conditions for the bacteria, modified methods of preparation of the enzyme. Acta Chem Scand. 1972;26(6):2401–2411. doi: 10.3891/acta.chem.scand.26-2401. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W., Costerton J. W., MacLeod R. A. Demonstration by freeze-etching of a single cleavage plane in the cell wall of a gram-negative bacterium. J Bacteriol. 1971 May;106(2):659–671. doi: 10.1128/jb.106.2.659-671.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Kishida Y., Olsen B. R., Berg R. A., Prockop D. J. Two improved methods for preparing ferritin-protein conjugates for electron microscopy. J Cell Biol. 1975 Feb;64(2):331–339. doi: 10.1083/jcb.64.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. C., Schmidt G., Jann K. Biochemistry of the K antigens of Escherichia coli. Formation of the nucleoside diphosphate sugar precursors of the K27 antigen of E. coli 08:K27(A):H-. Eur J Biochem. 1969 Dec;11(2):376–385. doi: 10.1111/j.1432-1033.1969.tb00783.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. The reversible dissociation of the alkaline phosphatase of Escherichia coli. 3. Properties of antibodies directed against the subunit. J Biol Chem. 1967 Apr 10;242(7):1599–1603. [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Studies on R mutants with an incomplete core, derived from E. coli O8:K27. Eur J Biochem. 1970 Oct;16(2):382–392. doi: 10.1111/j.1432-1033.1970.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Thompson L. M., MacLeod R. A. Biochemical localization of alkaline phosphatase in the cell wall of a marine pseudomonad. J Bacteriol. 1974 Feb;117(2):819–825. doi: 10.1128/jb.117.2.819-825.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Dvorak H. F., Heppel L. A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970 Oct;104(1):529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]