Abstract
Dendritic cells (DC) present antigenic epitopes to and activate T cells. They also polarize the ensuing T cell response to Th1 or Th2 type response, depending on their cytokine production profile. For example, IL-12 producing DC generate Th1 type T cell response whereas IL-10 producing DC is usually tolerogenic. Different strategies -- such as the use of cytokines and anti-cytokine antibodies, dominant negative forms of protein, anti-sense RNA etc. -- have been employed to influence the cytokine synthetic profile of DC as well as to make DC more immunogenic. Utilizing GFP expressing recombinant adenoviruses in association with lipid-mediated transfection of siRNA, we have silenced the endogenous IL-10 gene in DC. We show that IL-10 gene silenced DC produce more IL-12 and also generates a better cytolytic T cell response against the human melanoma associated epitope, MART-127−35, in-vitro. We also show that the GFP expressing adenoviral vector can be used to optimize the parameters for siRNA delivery in primary cells and show that RNA interference methodology can efficiently knock-down virus encoded genes transcribed at very high multiplicity of infection in DC.
Keywords: Dendritic cells (DC), Cytotoxic T Lymphocytes (CTL), Adenovirus, Interleukine- 10 (IL-10)
Introduction
As professional antigen presenting cells, Dendritic cells (DC) play a major role in the generation of an effective immune responses [1, 2]. Upon capturing antigens, DC migrate to the lymph nodes and present processed antigenic epitopes to T cells resulting in their activation [3, 4]. A variety of signals induce maturation DC that express high levels of antigen presenting and co-stimulatory molecules and certain cytokines critical for the nature of the T cell response. For example, Th1 type T cell responses need IL-12 synthesis by DC. DC can also produce immunosuppressive cytokines, such as IL-10 [5-7], which can influence the nature and the quality of the T cell response. IL-10 produced by DC can influence the DC maturation process, down-regulate IL-12 production and thus interfere with the Th1 type T cell response generation [3, 8-10]. IL-10 producing DC can also lead to immune tolerance [1]. Accordingly, there has been considerable interest in influencing the DC maturation process so as to skew T cell responses to a desired type (i.e., Th1 vs Th2/3) for translational purposes. Different strategies, such as using various cytokines, anti-cytokine antibodies, dominant negative forms of the proteins, and more recently through siRNA mediated gene silencing have been employed to modulate the DC phenotype [7, 11-15]. RNA interference (RNAi), the sequence specific degradation of target mRNAs by short double stranded RNAs of 21−23 nucleotide length [16, 17], has been successfully used in various mammalian cell lines as well as in primary cells such as T lymphocytes and antigen presenting cells [14, 17, 18]. Silencing of different cytokine genes has been used to generate desired immune responses [14, 15] and DNA vector, adenoviruses as well as lentiviruses have been successfully used to deliver siRNA in different cell systems [19-21]. Although siRNA technology has been effectively employed to silence different genes in APC, including IL-10, and modulate their phenotype [14, 15], to our knowledge, gene silenced DC has not been employed to generate a better CTL response against a relevant tumor antigen through active immunization or adoptive immunotheapy.
We show here that the delivery of siRNA oligonucleotides targeting GFP gene in combination with recombinant adenovirus expressing GFP protein can be used to optimize siRNA delivery conditions to achieve an effective gene silencing response in DC. Using optimized conditions, we show that the endogenous IL-10 gene in DC can be effectively silenced. We also show that IL-10 silenced DC up-regulate IL-12 production and result in the generation of a more robust CTL response against the human tumor associated antigenic epitope, MART-127−35. Additionally, we also show that genes expressed at extremely high viral load in dendritic cells (GFP in this case) can be effectively cleared utilizing siRNA technology.
Materials and Methods
Cell Lines, Culture Media, and Reagents
A375 (MART-1 negative human melanoma) [22] cells, obtained from the ATCC, were cultured in Dulbecco's modified Eagles` medium (DMEM) supplemented with 10% fetal bovine serum (FBS). A375-MG1, a constitutively GFP expressing A375 cell line was generated by transducing A375 cell line with GFP expressing retrovirus followed by serial dilution plating and selection of GFP positive clones. Peripheral blood derived DC were generated by Ficoll Hypaque gradient separation method as described before [23, 24]. Briefly, the peripheral blood lymphocytes were incubated in 6 well plates for 30 min. to 1 hour after which the non-adherent population was taken out and the adherent monocyte population was cultured in Iscoves Modified Dulbecco's Medium (IMDM) (Invitrogen, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gemini Bioproducts Inc., CA, USA), containing 1000u/ml recombinant human granulocyte macrophage colony - stimulating factor (GM-CSF) (Immunex, WA, USA) and 1000u/ml recombinant human interleukin (rhIL-4 (R& D Systems Inc., Minneapolis, USA), to differentiate them into DC.
Dendritic Cells Phenotype
Dendritic cells used in this study were phenotyped by quantifying the expression of CD80, CD86 and MHC Class I molecules before and after siRNA treatment by flowcytometry, as described previously [24, 25]. Briefly, 1× 105 cells were stained with antibodies against the desired molecule for 30−45 min., washed once with PBS, acquired using a FACS-Calibur and analyzed by Cell Quest software (Becton Dickinson, CA, USA), as reported previously [24, 25].
Adenovirus Mediated Delivery of GFP Protein in Dendritic Cells
Recombinant adenovirus vector expressing EGFP protein, used in this study to infect DC has been described previously [23]. The virus was amplified to higher titers in HEK 293 cells. The stock virus was cesium chloride gradient purified and titered on HEK 293 cells by adenovirus titration kit (BD Biosciences, CA, USA). For GFP silencing experiments in DC, cells were infected at 1000 moi (multiplicity of infection) virus, the dose found to be best to infect the DC monolayer as reported previously [23].
Si RNA Mediated Gene Silencing
Chemically synthesized siRNA oligos targeting EGFP and IL-10 genes were obtained from Dharmacon Inc., USA. The sequence of siRNA used for silencing GFP (Catalogue no. P-002102−01−20) is given below:
GFP-siRNA-: AAGACGTAAACGGCCACAAGTTC
IL-10 smart pool (Catalogue no. M-0050066−00) (Dharmacon Inc., IL, USA) was used to silence IL-10 gene. It contained a mixture of following four siRNA oligo-nucleotides:
IL-10-siRNA-1: TTAATAAGCTCCAGAGAA
IL-10-siRNA-2: TGGAGGACTTTAAGGGTTA
IL-10-siRNA-3: CATAGAAGCCTACATGACA
IL-10-siRNA-4: TCACGGCGCTGTCATCGAT
For gene silencing experiments, 3−5 days old immature DC were used. SiRNA were delivered in dendritic cells by conjugating with gene silencer transfection reagent (GenLantis Inc., CA, USA). Briefly, 5×105 immature DC were washed with PBS and re-suspended in 100μl serum free IMDM medium. SiRNA-lipid conjugates were prepared by adding the double stranded RNAs at different concentrations mentioned in the results section with 100μl serum free IMDM medium containing 10μl gene silencer reagent and incubated at room temperature for 15−20 minutes. SiRNA- lipid mixture was then co-incubated with DC at 37°C for four hours. After four hours, DC differentiating medium (IMDM containing GMCSF & IL-4) containing 2× FBS was added and DC were cultured at 37°C. GFP gene silencing was followed up to 6 days by immuno-fluorescence microscopy and also by quantification of GFP expression by FACS. For IL-10 gene silencing experiments, DC cultured for two days, post siRNA treatment, were analyzed for effective IL-10 gene silencing by RT-PCR analysis. DC culture supernatants were used for ELISA to measure the secreted IL-10 and IL-12 proteins.
RT-PCR
For the RT-PCR analysis, siRNA treated and control DC were lysed using the Tri reagent (Sigma Aldrich, MO, USA). Total RNA was isolated as per manufacturer's instruction and cDNA was prepared using the first strand cDNA synthesis kit (Invitrogen, CA, USA). PCR was performed for IL-10 (forward primer-GCCTAACATGCTTCGAGATC, reverse primer-CTCATGGCTTTGTAGATGCC), IL-12 (forward primer-GAAGGCCAGACAACTCTAG, reverse primer- CTATCAATAGCTACTGCCCG) and beta-actin (forward primer- GGCATCGTGATGGACTCCG, reverse primer- GTCGGAAGGTGGAC-AGCGA) genes and the PCR products were analyzed on 1.5% agarose gel.
In-vitro Cytotoxic T Lymphoctes (CTL) Generation Protocol
CTL generation experiments were essentially performed as described previously, with minor modification [23, 24, 26]. Two days post siRNA treatment, DC were matured by overnight treatment with LPS and IFN-γ as described previously [24]. Next day cells were harvested, counted to check the effect of siRNA treatment on DC survival and co-cultured with bead purified CD8+ve T cells, as described previously [23, 24]. Co-cultures were followed for at least 10 days before quantifying the expansion of MART-127−35 epitope specific CTL precursors by FACS analysis after staining the expanded T cells with MART-127−35 epitope specific tetramer, as described previously [23, 24]. Functional potential of expanded CTL was analyzed by measuring the amount of IFN-γ secretion following overnight co-culture with MART-127−35 peptide pulsed T2 cells, as described previously [25].
Quantification of siRNA Mediated GFP Silencing
GFP fluorescence in control and siRNA treated A375-MG1 cells and Adv-GFP infected DC were quantified by flow cytometry, as reported previously [24].
ELISA
IL-10, IL-12 and IFN-γ cytokines were measured by sandwich ELISA kit (Immunotech, Marseilles, France), according to the manufacturer's instruction.
Cytotoxicity Assay
Cytotoxic potential of CTL was measured by in-vitro chromium release assay, as described before [25]. Briefly, CTL generated with control matured DC (control CTL) and IL10 silenced matured DC (IL-10 si CTL), were co-cultured with Cr51 labeled, MART-127−35 peptide pulsed T2 cells or MART-1 positive human tumor cells, at different ratios, and percent specific lysis were calculated from the release of Cr51 in the culture supernatants [Percent Specific Lysis = (Test release – Spontaneous release) / (Total release – Spontaneous release) × 100].
Results
Tracking the delivery and effectiveness of GFP targeting siRNA oligonucleotide
We first checked the delivery and gene-silencing efficacy of the GFP targeting siRNA oligos by transfecting A375-MG1 cell line derived from A375 melanoma cell line by retrovirus-mediated integration of GFP gene. A375-MG1 cell line was transfected with gene silencer reagent coupled siRNA oligo, incubated for 48 hrs and analyzed by immunofluorescence microscopy. As shown in Fig.1A, GFP siRNA effectively knocked down the expression of GFP gene.
Figure. 1. SiRNA mediated silencing of GFP gene expression and quality of DC in siRNA experiments.
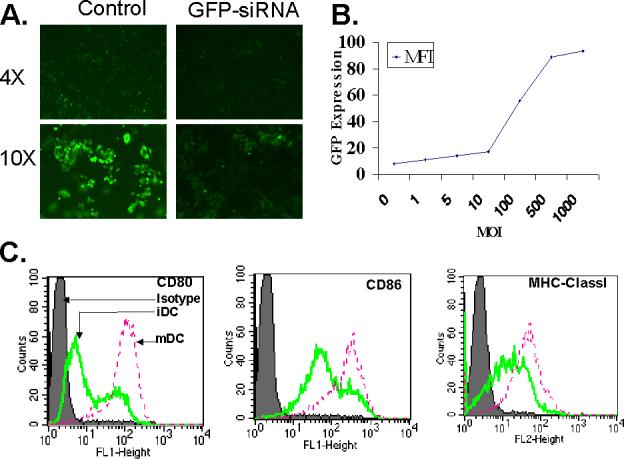
(A). Silencing of GFP gene in A375-MG1 cell line expressing GFP. GFP siRNA oligonucleotides (0.1 μM) was transfected in A375- MG1 cell line and GFP knockdown was examined 48 hr post transfection. (B). Adenoviral infection of dendritic cells. Dendritic cells were infected with GFP encoding recombinant adenovirus at different multiplicity of infections and the GFP expression was quantified by FACS analysis 48 hr post infection. (C). Phenotype of DC used for siRNA treatment and follow up experiments. DC used for siRNA treatment were phenotyped for the surface expression of MHC classI molecules and co-stimulatory molecules, CD80 and CD86 in immature state as well as following maturation with overnight LPS & IFN-γ treatment. All these experiments were done at least twice with similar results.
Optimization of siRNA delivery conditions in DC
We used both immature and fully matured myeloid DC for our studies. Adherent blood monocytes grown in GM-CSF and IL-4 were used as immature DC and when needed, they were further matured in LPS and interferon gamma treatment, a well established and routinely reported method for DC maturation [23, 24, 26]. Immature DC expressed lower levels of MHC class I molecules and CD80 & CD86 co-stimulatory molecules and these molecules were up regulated upon maturation (Fig. 1C).
Although lipid mediated delivery of siRNA has been routinely used to transfect cell lines, dendritic cells are usually difficult to transfect. In order to optimize the siRNA oligonucleotide delivery parameters such as the dose of lipid carrier, siRNA dose range and the time course of gene silencing etc., we utilized a recombinant adenovirus encoding GFP [23], in association with lipid mediated transfected siRNA specific for GFP mRNA. Optimum MOI of the recombinant adenovirus to infect DC was determined by infecting dendritic cells with Adv-GFP virus at various MOI shown in Fig.1B. Infected cells were observed under the microscope for GFP fluorescence and GFP expression levels were quantified by FACS analysis. MOI 1000 was found to be the optimum dose in accord with our previous observation [23]. All subsequent infections of DC were therefore carried out at 1000 MOI. For silencing of GFP mRNA transcripts, siRNA oligonucleotide specific for the GFP gene was used at doses mentioned in figure 2. The effect of siRNA treatment on GFP expression was observed under the microscope (Fig. 2B-C). In higher magnification micrographs, focus was deliberately put on areas showing GFP positive cells (Fig. 2B-C). However, after immunofluorescence micrograph analyses, these cells were analyzed by FACS to quantify the levels of GFP gene expression (Fig. 2D). As evident, significant suppression of GFP message was observed within 48 hrs of siRNA treatment and the gene silencing effect was stable up to six days at a dose range of 0.01 μM to 1.0 μM (Fig. 2D).
Figure. 2. Effect of siRNA targeting GFP gene on GFP expression in dendritic cells.
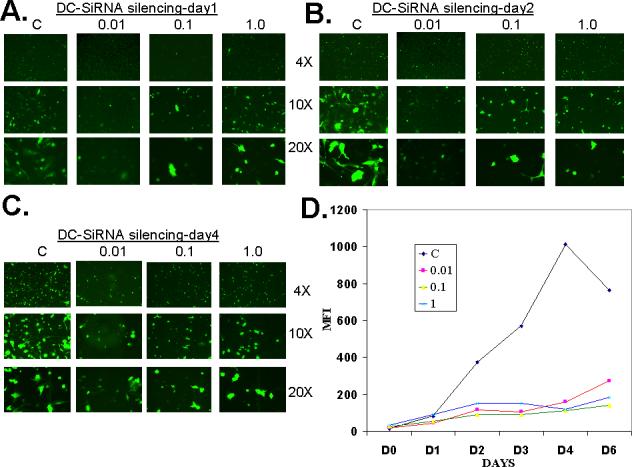
(A-C). Dendritic cells were transfected with 0.01, 0.1 and 1.0 μM siRNA oligos and GFP expression was examined under microscope from day 1 to day 6. (D). FACS analysis was done to quantify the GFP expression in GFP encoding recombinant adenovirus transduced DC and in DC transduced with recombinant virus and treated with 0.01, 0.1 and 1.0 μM siRNA, up to six days following treatment. These experiments were done twice with similar results.
Silencing of IL-10 in Dendritic Cells Results in Increased IL-12 Production and Up-regulation of Co-stimulatory Molecule Expression
In order to silence the IL-10 gene in DC, we used 0.1μM siRNA, which was found to be optimum for the longer suppression effect with GFP specific siRNA. The dose of 0.1μM IL-10 specific siRNA efficiently knocked down IL-10 both at the mRNA level (Fig. 3A) as well at the protein expression level (Fig. 3B).
Figure. 3. IL-10 gene silencing in dendritic cells results in higher IL-12 production.
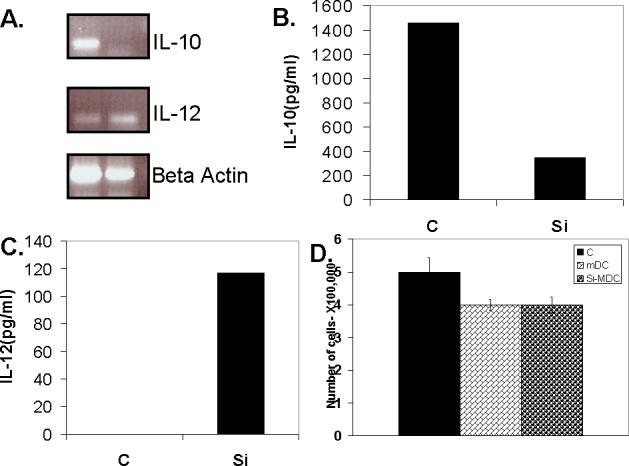
(A). RT PCR of Dendritic cells treated with IL-10 siRNA oligos (0.01 μM) show effective knockdown of IL-10 gene expression and resulted in increased IL-12 mRNA expression. (B & C). IL-10 (A) and IL-12 (B) ELISA on supernatants of dendritic cells treated with IL-10 siRNA show effective knockdown of IL-10 protein expression and resulting increase in IL-12 protein expression. (D). Effect of siRNA treatment on DC had no effect on their survival. siRNA treated immature DC were matured with LPS & IFN-γ and siRNA treated. Two days post treatment, DC were harvested and counted by trypan blue exclusion (C-control untreated DC, mDC-matured DC with no siRNA treatment, Si-mDC- matured and siRNA treated DC). These experiments were done three or more times with similar results.
Neuralization of endogenous IL-10 by anti-IL-10 antibody has been shown to increase IL-12 production by DC [7]. SiRNA has also been recently utilized to modulate DC biology and silencing IL-10 in DC has been shown to enhance Th1 response [15]. Silencing of IL-12 gene in DC has been shown to result in increased IL-10 production and Th2 cytokine production from allogenic T-cells [14]. We therefore examined the effect on IL-10 gene silencing on the DC phenotype. As shown in Figs. 3A & 3C, in agreement with previous observations [7, 15], IL-12 expression was indeed up-regulated in IL-10 silenced DC, both at the level of mRNA, analyzed by RT-PCR (Fig. 3A) as well as at protein level, measured by ELISA (Fig. 3C).
We checked the cytotoxic effect of the lipid treatment on DC. As shown in figure 3D, the siRNA treatment had no negative effect on the survival of DC. We further tested the effect of IL-10 gene silencing on the expression of co-stimulatory molecules. Fig. 4A shows that the siRNA treatment had no inhibitory effect on the DC surface expression of co-stimulatory molecules. In fact, in agreement with previous reports from other groups as well as our laboratory that IL-10 negatively affects expression of co-stimulatory molecules on DC [5-8], IL-10 knocked down DC had slightly higher levels of co-stimulatory molecules expression (Fig. 4A).
Figure. 4. IL-10 gene silenced DC results in moderately increased expression of co-stimulatory markers and generates a stronger CTL response against human melanoma associated antigenic epitope, MART-127−35.
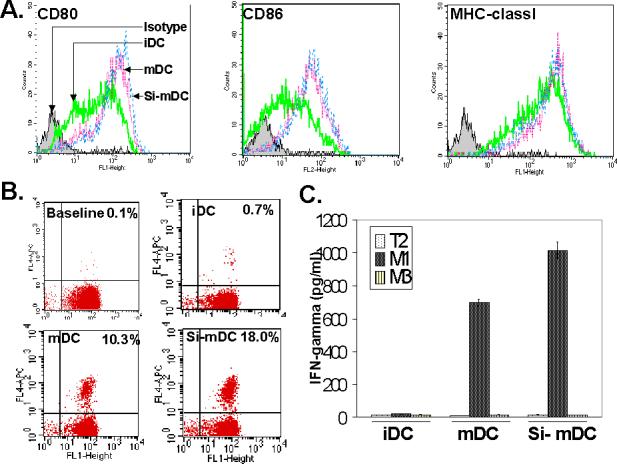
(A). Dendritic cells treated with siRNA were matured and phenotyped for the surface expression of co-stimulatory markers CD80, CD86 and MHC classI (iDC- immature DC, mDC- mature DC, Si-mDC-mature siRNA treated DC). (B). IL-10 silenced DC results in generation of a robust CTL response against human melanoma associated antigenic epitope, MART-127−35. In-vitro co-cultures of peripheral blood derived CD8 +ve T cells with monocyte derived immature DC, matured DC and matured and siRNA treated DC were set up and 10 days following co-culture, the expanded populations was examined for MART-127−35 epitope specific T cells by staining with CD8 (X-axis) and MART-127−35 specific tetramer (Y-axis) (C-Baseline CTL precursor frequency, iDC- in-vitro co-culture of CD8 +ve T cells with immature DC, mDC- in-vitro co-culture of CD8 +ve T cells with mature DC, Si-mDC-in-vitro co-culture of CD8+ve T cells with mature IL-10 silenced DC). (C). Quantification of IFN-γ secretion by MART-127−35 epitope specific CTL. The in-vitro expanded populations generated in Fig.4B were examined for IFN-γ production by exposing them overnight with either MART-127−35 peptide or control MAGE-3 control peptide pulsed T2 cells and doing ELISA on culture supernatants (iDC-immature DC co-cultured CTL population, mDC-mature DC co-cultured CTL population, Si-mDC-IL-10 silenced mature DC co-cultured CTL population). These experiments were done twice with similar results.
IL-10 silenced DC generates a stronger CTL response against the human melanoma associated epitope, MART-127−35
In order to check the immunogenic potential of IL-10 silenced DC, we used these DC to generate a tetramer epitope specific CTL response in our in-vitro CTL activation model against the human melanoma associated antigenic epitope, MART-127−35 [23, 24, 26]. As shown in Fig. 4B, IL-10 silenced mature DC resulted in the generation of a stronger CTL response in comparison with immature and mature DC. The tetramer stained CTL populations in both mature and IL-10 silenced mature DC were at located at identical heights in the FL4 upper right quadrant in Fig. 4B (tetramer staining axis), suggesting similar TCR affinity profiles of the two CTL populations. In order to quantify this CTL response functionally, we carried out ELISA for INF-γ with MART-127−35, peptide pulsed T2 cells. In accord with the increased MART-127−35 tetramer staining (Fig. 4B), CTL generated by IL-10 silenced DC correspondingly produced higher amounts of IFN-γ (Fig. 4C), thereby confirming that the expanded population generated by IL-10 silenced DC generated more antigen specific T cells. CTL generated with both types of DC (control matured DC and IL-10 silenced matured DC) exhibited similar cytokine release profile against different peptide dose range (data not shown), suggesting that qualitatively both CTL were somewhat similar. We also examined the cytolytic potential of the CTL generated against the two types of DC (Fig. 5). CTL generated from IL-10 silenced DC exhibited higher cytolytic potential against MART-127−35 peptide pulsed T2 cells, in an antigen specific manner (Fig. 5A). The CTL were also cytolytic against MART-1 positive human tumor cells (Fig. 5B).
Figure. 5. Analysis of cytolytic potential of CTL.
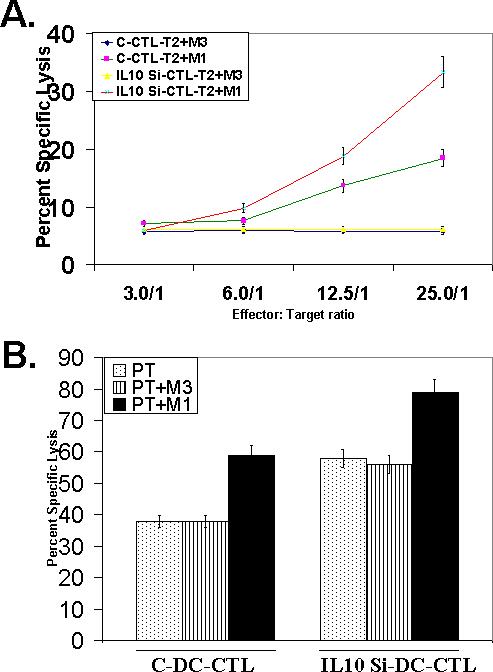
(A). CTL generated with control matured DC (C-CTL) and IL10 silenced matured DC (IL-10 Si-CTL), were co-cultured with Cr51 labeled, MART-127−35 cognate peptide pulsed (T2+M1) or control MAGE-3 control peptide pulsed T2 cells (T2+M3), at different ratios, and percent specific lysis were calculated from the release of Cr51 in the culture supernatants, as discussed in methods. (B). CTL generated with control matured DC (control CTL) and IL10 silenced matured DC (IL-10 si CTL), were co-cultured with Cr51 labeled, human melanoma derived, MART-1 positive tumor cells, PT, at 1:50 ratios, and percent specific lysis were calculated from the release of Cr51 in the culture supernatants, as discussed in methods. Cytotoxicity was also observed with PT cells pulsed with MART-127−35 peptide (PT+M1) or control MAGE-3 control peptide (PT+M3).
Discussion
Since DC can act both as facilitators and inhibitors of immune response, they are a good target for devising effective immunotherapeutic strategies [1, 27]. By presenting a given epitope, DC can be “immunogenic” as well as “tolerogenic” depending on the context in which the epitope is presented. From a translational viewpoint, considerable interest exists in influencing the nature of the immune response by modulating the DC phenotype. Altering the immune response towards the desired type (stimulatory vs inhibitory) is critical for the success of the treatment regimens employed against different conditions such as autoimmunity, organ transplantation and cancer.
It has been shown that the functional phenotype of DC can be influenced by interfering with the expression of important DC-tropic cytokine genes such as IL-10 and IL-12 [5, 6]. We have previously shown that even fully matured DC can synthesize IL-10 and such IL-10 producing DC can suppress T cell proliferation [5, 6]. Utilizing gene micro-array analysis of IL-10 treated DC we confirmed our initial observations and showed that IL-10 treatment does indeed down-regulates the gene expression of IL-12 and co-stimulatory molecules in human DC [25]. Our findings have also been independently confirmed by other groups [7] and it has been shown that exogenous IL-10 treatment of monocytes results in down-regulated expression of co-stimulatory molecules [5-7] and such treatment can affect the generation of Th1 type of immune responses [10].
Generation of DC with superior immunogenic potential is essential for developing effective immunotherapeutic strategies against infectious agents and cancers. For an effective cancer immunotherapy, generation of a robust and strong CTL response against tumor-associated antigens in vivo by DC based active immunization or in-vitro for the purpose of adoptive immunotherapy is critical [28-31]. Various laboratories including our`s have utilized different strategies, to achieve a stronger and long lasting CTL response against cancer antigens [2, 23, 26, 27, 30, 32]. Among various approaches we have employed include improving antigen delivery and processing conditions in DC [23] and interfering with the activation induced cell death (AICD) in the expanded CTL to sustain the CTL response longer [23, 26]. However, since one of the most important limiting factor in generating an effective CTL response is the immunogenic potential of DC [33], devising strategies to generate DC with superior immunostimulatory phenotype, such as we have shown here is noteworthy and will be useful. Our data show that RNAi mediated ablation of inhibitory DC effectors such as IL-10 can generate high quality DC with superior stimulatory phenotype which can eventually generate a stronger CTL response against tumor associated antigens.
Although neutralization of DC synthesized IL-10 by IL-10-neutralizing antibody has been shown to overcome the inhibitory nature of DC and to lead to higher secretion of IL-12 and increased expression of co-stimulatory markers [5-7], siRNA based knock-down of endogenous immunosuppressive agents will be a preferred method to make DC more effective for therapeutic strategies such as active immunization. Recently, siRNA mediated ablation of IL-10 in human DC has been shown to enhance Th1 type of T cell responses by CD4+ effector T cells [15]. However, to our knowledge, IL-10 silenced DC has not been utilized to generate a better antigen specific CTL response against relevant system human tumor antigens. Our data extend the basic findings of Liu et al [15], on CD8+ CTL in a human tumor antigen model. We show that the IL-10 silenced DC not only produce more IL-12 (Fig. 3), they also generate a CTL response of a greater magnitude to a clinically relevant tumor antigenic epitope, in-vitro (Fig. 4B). Since the CTL populations generated with both DC types (control matured DC and IL-10 silenced matured DC) exhibited similar IFN-γ release profile over different peptide dose range (data not shown), the two CTL populations were apparently similar, qualitatively. As such, it can be argued that the IL-10 silenced DC did not influence TCR “affinity/avidity“ of expanded CTL. The IL-10 silenced DC, nonetheless, had a positive effect on the expansion of the epitope specific CTL. The greater magnitude of the CTL response generated with the IL-10 silenced DC was evident by the higher percent tetramer positive populations along with a corresponding higher secretion of IFN-γ (Fig. 5C) and increased cytotoxicity against MART-127−35 peptide positive tumor and non-tumor cells (Fig. 5).
Finally, it should be pointed out that the methodology we have used here to track the siRNA oligo delivery by transducing human dendritic cells with adenovirus encoding GFP gene (Figs. 1A & 2) can also be effectively utilized to optimize the effective parameters such as the dose of lipid carrier, effective siRNA dose range and the time course of gene silencing etc., in otherwise difficult to transfect primary cells. Our data also suggest that the siRNA technology can be an effective anti-viral treatment, given the fact that a relatively high titer of transduced adeno-viral encoded GFP mRNA transcripts were effectively knocked down in DC (Fig. 2). Admittedly, knocking down of adenovirus encoded GFP gene does not replicate adenovirus infection, but our data suggest that siRNA mediated targeting of viral genes essential for their replication and/or packaging, can be effectively used for siRNA- based control of viral infection in DC, as has been shown in different systems [34, 35]. Collectively, our findings have implications in siRNA based therapeutics as well as in DC-based immunotherapy.
Acknowledgments
The work was supported by PHS grants CA 83130 and CA 88059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Fay J, Paczesny S, Ueno H, Dhodapkarz M, Palucka AK. Dendritic cells as melanoma vaccines. Dev Biol (Basel) 2004;116:147. [PubMed] [Google Scholar]
- 3.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13(5):275. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 4.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1(3):199. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty A, Li L, Chakraborty NG, Mukherji B. Stimulatory and inhibitory maturation of human macrophage-derived dendritic cells. Pathobiology. 1999;67(5−6):282. doi: 10.1159/000028080. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty A, Li L, Chakraborty NG, Mukherji B. Stimulatory and inhibitory differentiation of human myeloid dendritic cells. Clin Immunol. 2000;94(2):88. doi: 10.1006/clim.1999.4826. [DOI] [PubMed] [Google Scholar]
- 7.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166(7):4312. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 8.Willems F, Marchant A, Delville JP, Gerard C, Delvaux A, Velu T, de Boer M, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24(4):1007. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 9.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the generation of dendritic cells from CD14+ blood monocytes, promotes the differentiation to mature macrophages and stimulates endocytosis of FITC-dextran. Adv Exp Med Biol. 1997;417:323. doi: 10.1007/978-1-4757-9966-8_53. [DOI] [PubMed] [Google Scholar]
- 10.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27(5):1229. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Rich BE, Inobe J, Chen W, Weiner HL. Induction of Th2 cell differentiation in the primary immune response: dendritic cells isolated from adherent cell culture treated with IL-10 prime naive CD4+ T cells to secrete IL-4. Int Immunol. 1998;10(8):1017. doi: 10.1093/intimm/10.8.1017. [DOI] [PubMed] [Google Scholar]
- 12.Letterio JJ, Bottinger EP. TGF-beta knockout and dominant-negative receptor transgenic mice. Miner Electrolyte Metab. 1998;24(2−3):161. doi: 10.1159/000057365. [DOI] [PubMed] [Google Scholar]
- 13.Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23(11):1399. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- 14.Hill JA, Ichim TE, Kusznieruk KP, Li M, Huang X, Yan X, Zhong R, Cairns E, Bell DA, Min WP. Immune modulation by silencing IL-12 production in dendritic cells using small interfering RNA. J Immunol. 2003;171(2):691. doi: 10.4049/jimmunol.171.2.691. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Ng H, Akasaki Y, Yuan X, Ehtesham M, Yin D, Black KL, Yu JS. Small interference RNA modulation of IL-10 in human monocyte-derived dendritic cells enhances the Th1 response. Eur J Immunol. 2004;34(6):1680. doi: 10.1002/eji.200425081. [DOI] [PubMed] [Google Scholar]
- 16.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101(1):25. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 17.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes Dev. 2001;15(2):188. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McManus MT, Haines BB, Dillon CP, Whitehurst CE, van Parijs L, Chen J, Sharp PA. Small interfering RNA-mediated gene silencing in T lymphocytes. J Immunol. 2002;169(10):5754. doi: 10.4049/jimmunol.169.10.5754. [DOI] [PubMed] [Google Scholar]
- 19.Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci U S A. 2002;99(8):5515. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen C, Buck AK, Liu X, Winkler M, Reske SN. Gene silencing by adenovirus-delivered siRNA. FEBS Lett. 2003;539(1−3):111. doi: 10.1016/s0014-5793(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 21.Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003;100(4):1844. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai Y, Yang JC, Kawakami Y, Spiess P, Wadsworth SC, Cardoza LM, Couture LA, Smith AE, Rosenberg SA. Antigen-specific tumor vaccines. Development and characterization of recombinant adenoviruses encoding MART1 or gp100 for cancer therapy. J Immunol. 1996;156(2):700. [PubMed] [Google Scholar]
- 23.Chhabra A, Mehrotra S, Chakraborty NG, Mukherji B, Dorsky DI. Cross-presentation of a human tumor antigen delivered to dendritic cells by HSV VP22-mediated protein translocation. Eur J Immunol. 2004;34(10):2824. doi: 10.1002/eji.200425192. [DOI] [PubMed] [Google Scholar]
- 24.Mehrotra S, Chhabra A, Chattopadhyay S, Dorsky DI, Chakraborty NG, Mukherji B. Rescuing melanoma epitope-specific cytolytic T lymphocytes from activation-induced cell death, by SP600125, an inhibitor of JNK: implications in cancer immunotherapy. J Immunol. 2004;173(10):6017. doi: 10.4049/jimmunol.173.10.6017. [DOI] [PubMed] [Google Scholar]
- 25.Mehrotra S, Chhabra A, Chakraborty A, Chattopadhyay S, Slowik M, Stevens R, Zengou R, Mathias C, Butterfield LH, Dorsky DI, Economou JS, Mukherji B, Chakraborty NG. Antigen presentation by MART-1 adenovirus-transduced interleukin-10-polarized human monocyte-derived dendritic cells. Immunology. 2004;113(4):472. doi: 10.1111/j.1365-2567.2004.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhabra A, Mehrotra S, Chakraborty NG, Dorsky DI, Mukherji B. Activation-induced cell death of human melanoma specific cytotoxic T lymphocytes is mediated by apoptosis-inducing factor. Eur J Immunol. 2006;36(12):3167. doi: 10.1002/eji.200636550. [DOI] [PubMed] [Google Scholar]
- 27.Nouri-Shirazi M, Banchereau J, Fay J, Palucka K. Dendritic cell based tumor vaccines. Immunol Lett. 2000;74(1):5. doi: 10.1016/s0165-2478(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 28.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99(25):16168. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherji B, Chakraborty NG, Yamasaki S, Okino T, Yamase H, Sporn JR, Kurtzman SK, Ergin MT, Ozols J, Meehan J, et al. Induction of antigen-specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide-pulsed autologous antigen presenting cells. Proc Natl Acad Sci U S A. 1995;92(17):8078. doi: 10.1073/pnas.92.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yee C, Riddell SR, Greenberg PD. Prospects for adoptive T cell therapy. Curr Opin Immunol. 1997;9(5):702. doi: 10.1016/s0952-7915(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celis E. Getting peptide vaccines to work: just a matter of quality control? J Clin Invest. 2002;110:1765. doi: 10.1172/JCI200217405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, Nandamuri KM. Inhibition of hepatitis viral replication by siRNA. Expert Opin Biol Ther. 2004;4(10):1649. doi: 10.1517/14712598.4.10.1649. [DOI] [PubMed] [Google Scholar]
- 35.Morris KV, Rossi JJ. Lentiviral-mediated delivery of siRNAs for antiviral therapy. Gene Ther. 2006;13(6):553. doi: 10.1038/sj.gt.3302688. [DOI] [PMC free article] [PubMed] [Google Scholar]


