Abstract
Pontine noradrenergic neurons of the locus coeruleus (LC) and sub-coeruleus (SubC) region cease firing during rapid eye movement sleep (REMS). This plays a permissive role in the generation of REMS and may contribute to state-dependent modulation of transmission in the central nervous system. Whether all pontomedullary catecholaminergic neurons, including those in the A1/C1, A2/C2 and A7 groups, have REMS-related suppression of activity has not been tested. We used Fos protein expression as an indirect marker of the level of neuronal activity and linear regression analysis to determine whether pontomedullary cells identified by tyrosine hydroxylase (TH) immunohistochemistry have reduced Fos expression following REMS-like state induced by pontine microinjections of a cholinergic agonist, carbachol in urethane-anesthetized rats. The percentage of Fos-positive TH cells was negatively correlated with the cumulative duration of REMS-like episodes induced during 140 min prior to brain harvesting in the A7 and rostral A5 groups bilaterally (p<0.01 for both), and in SubC neurons on the side opposite to carbachol injection (p<0.05). Dorsal medullary A2/C2 neurons did not exhibit such correlation, but their Fos expression (and that in A7, rostral A5 and SubC neurons) was positively correlated with the duration of the interval between the last REMS-like episode and the time of sacrifice (p<0.05). In contrast, neither of these correlations was significant for A1/C1 or caudal A5 neurons. These findings suggest that, similar to the prototypic LC neurons, neurons of the A7, rostral A5 and A2/C2 groups have reduced or abolished activity during REMS, whereas A1/C1 and caudal A5 neurons do not have this feature. The reduced during REMS activity in A2/C2, A5 and A7 neurons, and the associated decrements in norepinephrine release, may cause state-dependent modulation of transmission in brain somato- and viscerosensory, somatomotor, and cardiorespiratory pathways.
Keywords: autonomic regulation, brainstem, locus coeruleus, motor control, norepinephrine
Pontomedullary catecholaminergic (CA) neurons are important for cardiorespiratory regulation (Chan and Sawchenko, 1994; Dampney et al., 2003; Schreihofer and Guyenet, 2000; Guyenet, 2006), as well as somato- and viscerosensory transmission (Blessing et al., 1982; Ross et al., 1985; Morilak and Jacobs, 1985). Noradrenergic (NE) cells of the locus coeruleus (LC) and subcoeruleus (SubC) region become silent during rapid eye movement sleep (REMS) (Aston-Jones and Bloom, 1981; Reiner, 1986). Similarly, in anesthetized rats, both LC neurons and pontine A5 neurons are silenced during REMS-like state induced by pontine microinjections of a cholinergic agonist, carbachol (Kubin, 2001; Fenik et al., 2002). The reduced activity of brainstem NE neurons plays a permissive role in the generation of REMS (reviewed by Siegel, 1994; Jones, 2004), and silencing of NE neurons during REMS, along with a concomitant reduction of norepinephrine release, may diminish excitatory drive to motoneurons, thereby causing the atonia characteristic of REMS (Lai et al., 2001; Fenik et al., 2005; Chan et al., 2006). The activity of LC neurons, which is reduced during slow-wave sleep and abolished during REMS, may represent a prototypic pattern typical of most, or all, brainstem CA neurons, but whether this is the case has not been tested.
Expression of Fos, a protein product of the immediate-early gene c-fos, has been used as a marker of cellular activation in relation to sleep-wake states (Yamuy et al., 1993; Cirelli et al., 1995; Merchant-Nancy et al., 1995; Shiromani et al., 1995; Sherin et al., 1996; Basheer et al., 1997; Cirelli and Tononi, 2000). In some studies, immunohistochemistry for Fos was combined with labeling of different neuronal phenotypes (Maloney et al., 1999; Verret et al., 2005; Gvilia et al., 2006). Most of those studies pointed to a good agreement between the levels of Fos expression and cell firing rates across different sleep-wake states, including REMS.
In the present study, we used Fos immunohistochemistry to examine the behavior of brainstem CA neurons in relation to REMS-like state elicited in urethane-anaesthetized rats by pontine microinjections of carbachol. In this model, one can repeatedly elicit REMS-like episodes comprising cortical and hippocampal activation, silencing of brainstem aminergic cells and suppression of motoneuronal activity (Kubin, 2001; Fenik et al., 2002; Kubin and Fenik, 2004). The episodes likely involve cellular and network activity that, albeit simplified, bears major similarities to that during natural REMS in behaving animals (Kubin, 2001; Fenik et al., 2002; Fenik et al., 2005; Lu et al., 2007). We found that Fos expression in SubC and A5 neurons was negatively correlated with the cumulative duration of REMS-like state in accordance with earlier single-cell recording studies showing that these cells cease firing during REMS. We then obtained new evidence suggesting that A7 and A2/C2, but not A1/C1 cells, also have reduced activity during REMS-like state. Preliminary results have been published (Rukhadze et al., 2007).
EXPERIMENTAL PROCEDURES
Experiments were performed on 17 adult, male, Sprague-Dawley rats (body weight: 396 g ±6.8 (SE) obtained from Charles River Laboratories (Wilmington, MA). All animal handling procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Animal preparation
The animals were pre-anesthetized with isoflurane (2%) followed by urethane (1.0 g•kg, i.p.). The trachea was cannulated and catheters inserted into the femoral artery and vein for arterial blood pressure monitoring and fluid injections, respectively. Under hypothermic conditions (~33 °C), the right XII nerve was cut peripherally and placed in a cuff-type recording electrode (Fenik et al., 2001). Both vagi were cut in the neck to enhance XII nerve activity and make it independent of lung volume feedback. The animal was placed in a stereotaxic head holder and two openings were made in the right parietal bone for inserting a carbachol-containing pipette into the pontine reticular formation and a bipolar recording electrode into the hippocampus. The cortical EEG, hippocampal activity and XII nerve activity were monitored, as previously described (Fenik et al., 2005).
The arterial blood pressure, rectal temperature and end-expiratory CO2 (Columbus Instruments capnograph, Columbus, OH) were continuously monitored. The animals were paralyzed (pancuronium bromide, 2 mg•kg−1 i.v., supplemented with 1 mg•kg−1 injections as needed) and artificially ventilated with an air-oxygen mixture (30–60% O2) at 50–70 lung inflations/min. Following paralysis, the level of anesthesia was judged to be adequate based on the presence of stable respiratory rhythm, arterial blood pressure, EEG, hippocampal activity and XII nerve activity. The level of anesthesia was titrated to maintain stable conditions by supplemental doses of the anesthetic applied i.v. in 50 mg increments. Recordings were obtained from animals with the mean systolic arterial blood pressure of 98±4.8 mmHg.
Electrophysiological recordings
Cortical (bandwidth 1–100 Hz), hippocampal (2–20 Hz) and XII nerve (30–2500 Hz) activities were recorded with AC amplifiers (N101, Neurolog System; Digitimer, Hertfotdshire, UK). All signals were monitored on an 8-channel chart recorder (TA-11; Gould Instruments, Valley View, OH) and recorded using a 16-channel digital tape recorder (C-DAT; Cygnus Technology, Delaware Water Gap, PA). To monitor changes in the magnitude of inspiratory modulation of XII nerve activity, the activity was full-wave rectified and passed through a moving average circuit (time constant 100 ms; MA-821 RSP; CWE, Inc., Ardmore, PA).
Microinjections
In 14 animals, 10 nl injections of carbamylcholine chloride (carbachol, 10 mM in saline, Sigma, St. Louis, MI) were made into the dorsomedial pontine reticular formation at sites where the drug effectively elicits REMS-like episodes (Kubin, 2001; Fenik et al., 2005). In additional three animals, only saline (10 nl) was injected multiple times into the same pontine region. Pontamine sky blue dye (2%, ICN Biomedicals Inc., Aurora, OH) was added to the injected solutions to mark the injection sites. The injections were made using glass pipettes (A-M Systems, Carlsborg, WA) with tip diameter 25–30 μm over 10–20 s by applying pressure to the fluid in the pipette and monitoring the movement of the meniscus through a calibrated microscope.
Experimental protocol
Once the animal was prepared for recording, carbachol pipette was placed into the dorsomedial pontine reticular formation and a 10 nl carbachol injection was made to verify its effectiveness at the selected site; in five out of the 14 animals with carbachol injections, the position of the pipette had to be adjusted one or two times to obtain the desired REMS-like effect. During the subsequent 2 h, the temperature of the animal was elevated to 35.5–36.5 °C and then maintained constant with a servo-controlled heating pad. The end-expiratory CO2 was set at 5.5–6.0% to obtain steady respiratory modulation of XII nerve activity and was kept constant throughout the experiment. Once all conditions were stabilized, 1–12 carbachol injections or 5–6 saline injections were made during a fixed period of 5 h after which the animal was perfused. The total duration of REMS-like episodes generated during this period ranged from 3.2 to 71.9 min (mean: 22.9±5.8). The animals were perfused on the average 47.2±7.5 min (range: 8–94 min) after the last REMS-like episode, or 15.5±0.9 min (range: 14–17 min) after the last saline injection.
Histological and immunohistochemical procedures
The experiments were concluded with administration of an additional dose of urethane (1g/kg) immediately followed by intra-arterial perfusion with phosphate-buffered saline (PBS, pH7.4, with 5 USP units/ml of heparin and 0.004% lidocaine, 4–6 °C) and then by 4% phosphate-buffered formalin. The pons (all 17 animals), or both the pons and the medulla (13 of those animals), was removed, postfixed in the same fixative, cryoprotected in 30% sucrose and cut into five series of 35 μm coronal sections. Selected sections containing the blue dye were mounted and stained with neutral red to localize the injection site. Sections from one complete series were processed as previously described by Lu et al. (2007). They were successively incubated with antibodies for c-Fos (1:100,000; Lot number D14211; Oncogene, Temecula, CA) and then for tyrosine hydroxylase (TH, 1:35,000; Lot number 41K4829; Sigma). Incubation with each primary antibodies was followed by appropriate biotinylated secondary antibodies, then an avidinbiotin reaction (Vector, Burlinghame, CA), and then a diaminobenzidine-horseradish peroxidase reaction. The latter was heavy metal-intensified for Fos, resulting in a black reaction product, but was conducted without intensification for TH, resulting in a golden-brown reaction product. Photomicrographs of stained cells were taken using an upright microscope (Leica DML, Wetzlar, Germany) and a digital camera (DMC Ie, Polaroid, Cambridge, MA). Image processing was limited to brightness and contrast adjustments to most faithfully represent the appearance of the specimens, as seen under direct microscopic observation (Photoshop CS software, Adobe, San Jose, CA).
Cell counting
For cell counting, sections were observed at 200x magnification. All TH-positive cells, with and without Fos-positive nuclei, were counted bilaterally in every 5th section in medullary A1/C1, A2/C2 and pontine A5, A7 and SubC regions. In the A2/C2 region, cells were counted in the nucleus of the solitary tract only, whereas other TH-positive cell bodies located in the area postrema (AP) or the dorsal motor nucleus of vagus were excluded, because some of them are dopaminergic (Armstrong et al., 1982; Hökfelt et al., 1984; Kalia et al., 1985). The A1/C1 region was divided into three sub-regions: a predominantly noradrenergic A1 region located at levels caudal to the area postrema (AP-14.6 to AP-14.3, according to a rat brain atlas of Paxinos and Watson (1997), an intermediate (mixed-adrenergic/noradrenergic) A1/C1 region located at the level of the area postrema (AP-14.08 to AP-13.68), and predominantly adrenergic C1 region located rostral to the area postrema (AP-13.3 to AP-11.6) (Armstrong et al., 1982; Hökfelt, 1984). We also separately counted A5 cells in the caudal (from AP-11.00 to just caudal to AP-9.8) and rostral (from AP-9.8 to AP-8.8) regions (cf., Lyons and Grzanna, 1988; Clark and Proudfit, 1993). The cells of the LC proper were not analyzed because reliable cell counts could not be obtained from the LC due to extremely high NE cell density and strong TH staining characteristic of this nucleus that, in some cells, obscured reliable recognition of Fos staining.
The percentages of Fos-positive CA neurons were calculated within each group relative to all TH-positive cells in that group. To asses the effect of carbachol or saline on Fos expression in the region adjacent to the injection site, Fos-positive nuclei were counted at 100x magnification within a 1×1 mm box centered around the injection site and in a counting box of the same size symmetrically placed on the opposite side of the pons.
Signal processing and data analysis
Cortical and hippocampal activities were digitally acquired off-line using Spike-2 software (Cambridge Electronics Design, Cambridge, UK; sampling rate 100 Hz) and then analyzed using a commercial sleep scoring software (Somnologica; MedCare, Buffalo, NY). Absolute changes in spectral powers of the EEG and hippocampal signal were calculated in distinct frequency bands in successive 10 or 15 s intervals during baseline and REMS-like responses; for normalization, they were divided by the total spectral power in 0.1–15 Hz range recorded under the baseline condition.
Statistical analyses were performed using linear regression analyses and two-tailed t-test. Spectral powers were analyzed using two-way repeated measures analysis of variance (ANOVA) with the effect of state and state triggering event (carbachol or “spontaneous”), as the two factors (Sigma Plot and Sigma Stat, Jandel, San Rafael, CA). Differences were considered significant when p<0.05. The variability of the means is characterized by the standard error (SE).
RESULTS
Temporal distribution and cumulative duration of REMS-like episodes
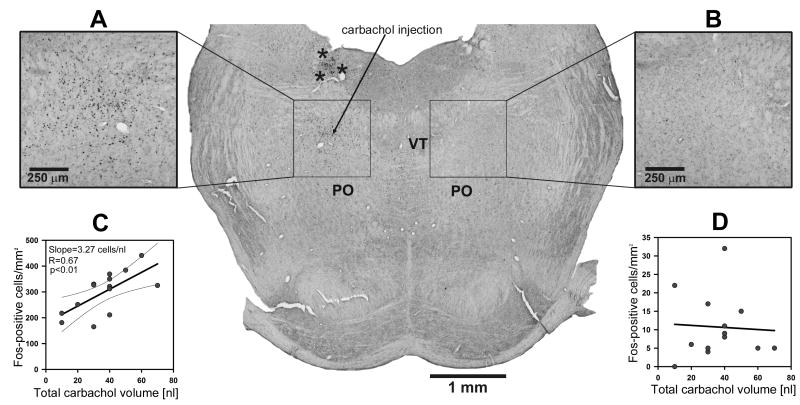
Variable numbers of carbachol or saline injections were made to obtain different cumulative durations of REMS-like state during the 5 h period of the experimental protocol. The injections were placed at the anterior-posterior levels from AP-8.1 to AP-8.9 caudal to bregma (Paxinos and Watson, 1997), within the dorsomedial pontine reticular formation region that we previously determined to be effective in eliciting REMS-like episodes (Kubin, 2001; Fenik et al., 2005; Lu et al., 2007). Figure 1 shows the locations of the centers of all injections superimposed on two closest standard brain sections from a rat brain atlas (Paxinos and Watson, 1997).
Figure 1.
Distribution of carbachol (filled circles) and saline (triangles) injection sites within the dorsomedial pontine reticular formation in the 17 rats used in this study. The centers of all injection sites are superimposed on two closest standard sections taken from levels B-8.3 and B-8.8 mm from bregma of a rat brain atlas (Paxinos and Watson, 1997). PO – nucleus pontis oralis; scp – superior cerebellar peduncle; VT – ventral tegmental nucleus.
As described previously (Kubin and Fenik, 2004; Fenik et al., 2005; Lu et al., 2007), carbachol-induced REMS-like episodes were characterized by activation of the cortical EEG in 6–12 Hz range, the appearance of hippocampal theta-like rhythm (3–4 Hz in anaesthetized rats), decreased respiratory rate and suppression of XII nerve activity. The episodes occurred within 59.7±8.6 s (range: 2.4–390 s) following the onset of carbachol injections, and their mean duration was 4.5±0.4 min (range: 1–13 min). One example of a carbachol-triggered REMS-like episode is shown in Fig. 2A.
Figure 2.
Examples of two REMS-like episodes, one carbachol-elicited (A) and one spontaneously generated (B), recorded from the same animal. The spontaneous episode occurred 30 min after a carbachol-induced one without being preceded by carbachol injection for 15 min. The episodes had similar patterns and magnitudes in all measured outputs (hippocampal and cortical powers, depression of XII nerve activity and reduction of respiratory rate), suggesting that they were generated by the same neuronal network. The records are from the animal number 11 in Fig. 3.
In eight of the 14 carbachol-injected animals, episodes having the patterns and durations closely resembling carbachol-induced REMS-like episodes occurred spontaneously, i.e., without being preceded by a carbachol injection for at least 15 min. Figure 2B shows an example of a “spontaneous” REMS-like episode in the same animal from which a carbachol-triggered episode is illustrated in Fig. 2A. By visual inspection, both episodes occurred with similar changes in the respiratory rate and XII nerve activity. A quantitative comparison of EEG and hippocampal changes during the spontaneous and carbachol-triggered REMS-like episodes was conducted using data from three animals from which tape recordings of four episodes of both types were available. The normalized EEG power in 6–12 Hz range increased from 5.72% ±0.9 to 13.8% ±1.6 during the carbachol-induced REMS-like episodes, and from 5.80% ±0.98 to 12.8% ±1.7 during the spontaneous episodes recorded in the same animals. The hippocampal power in the 3–5 Hz range (theta) increased from 20.0% ±1.3 to 150% ±56 during the carbachol-induced REMS-like episodes, and from 24.1% ±4.3 to 234% ±61 during the spontaneous episodes. Two-way repeated measures ANOVA revealed that the increases in both EEG (F1,15=19.1, p<0.05) and hippocampal (F1,15=15.2, p<0.05) powers were significant during both the carbachol-induced and spontaneous REMS-like episodes, whereas the magnitudes of power increase during the carbachol-induced and spontaneous REMS-like episodes were not significantly different for either the EEG (F1,3=2.85) or hippocampal theta-like activity (F1,3=1.35). There was also no interaction between the magnitude of the increase in EEG or hippocampal powers and the type of REMS-like episode (F1.3=4.59 for the EEG, and F1.3=1.17 for hippocampal activity). Since no differences were found, the carbachol-induced and spontaneous episodes were combined for the purpose of calculation of the cumulative durations of REMS-like state prior to animal sacrifice.
In some animals, only one or two carbachol injections were made, whereas in other rats, carbachol was injected as frequently as every 15–20 min to maximize the resulting time spent in REMS-like state (when injections were made frequently, not all of them were effective in triggering a REMS-like episode). Figure 3 shows the temporal distribution of all carbachol and saline injections and all REMS-like episodes that occurred in different animals during the entire 5 h period prior to animal sacrifice (at time zero). The experiments are listed in the order of decreasing cumulative duration of REMS-like episodes (experiments with saline injections at the bottom). The length of the bars represents the duration of individual episodes. The spontaneous REMS-like episodes are those that are not immediately preceded by filled triangles that mark carbachol injections. The numbers on the right show, for each animal, the total duration of REMS-like state during the entire 5 h period of the experimental protocol and also during the last 140 min prior the end of the experiment, a period that we found optimal for data analysis (next section).
Figure 3.
Temporal distribution of pontine carbachol and saline injections and periods of occurrence of REMS-like episodes during 5 h prior to sacrifice in individual animals. The length of the bars represents the duration of REMS-like episodes. The bars immediately preceded by filled triangles correspond to carbachol-triggered REMS-like episodes and those not preceded by triangles represent REMS-like episodes that occurred spontaneously. The triangles that are not followed by bars show carbachol injections that were not effective in triggering REMS-like effects, and open diamonds show saline injections. The two columns of numbers on the right list the total durations of REMS-like episodes in individual animals during the entire 5 h of the experimental protocol and during the last 140 min prior to sacrifice, which was the time used in all analyses.
Correlation of Fos expression in CA neurons with the cumulative duration of REMS like-episodes occurring during different periods prior to sacrifice
In earlier studies in which the magnitude of Fos expression was correlated with time spent in different behavioral states, periods of observations prior to sacrifice ranged from 1 to 3 h (Shiromani et al., 1995; Basheer et al., 1997; Maloney et al., 1999; Verret et al., 2005). However, the rationale for selection of the observation period is not well established because the data about the time course of Fos accumulation and dissipation in different cell types and brain regions in vivo is scarce. To establish rational basis for selection of an optimal period of observation prior to sacrifice, we assessed the strength of the regression between Fos expression in TH neurons and the cumulative duration of REMS-like episodes for different observation periods ranging from 5 to 1 h. The analysis was based on Fos counts in A7 neurons located opposite to the carbachol injection site because preliminary analysis revealed that these neurons yielded most robust correlations among all CA groups.
Figure 4 shows the relationship between the period prior to sacrifice during which the total duration of REMS-like state was considered and the resulting absolute value of the linear regression coefficient. For all periods between 300 and 200 min prior to sacrifice, the correlations were statistically significant with a nearly constant value of the regression coefficient. The coefficient increased for shorter periods of analysis, i.e. the strength of the regression improved slightly, reached a maximum around 140 min and then rapidly declined. This outcome suggested that REMS-like episodes that occurred more than ~140 min prior to sacrifice had relatively smaller effect on Fos expression in A7 neurons than those occurring during the last 140 min preceding the sacrifice. Accordingly, we chose to analyze our data in relation to the cumulative duration of REMS-like episodes that occurred during 140 min prior to sacrifice.
Figure 4.
Negative correlation between the percentage of A7 cells expressing Fos and cumulative duration of REMS-like state was stronger for the analysis based on the last ~140 min prior to sacrifice than for any other period. Linear regression coefficients were separately calculated for different periods prior to sacrifice. Data points between −190 min and 7minus;80 min were fit with a parabolic equation to estimate the position of the maximum. Based on this analysis, 140 min was selected as an optimal period for correlation of Fos expression with the amount of prior REMS-like state.
Effect of carbachol on Fos expression near the injection site
To determine whether carbachol injections activated neurons located near the injection site, and whether this activation was proportional to the resulting amount of REMS-like state, we counted Fos-positive nuclei in the one section located closest to the injection site in each animal. Fos-positive nuclei were very numerous on the injected side but few in the symmetrically located region on the opposite side. In the 14 rats with carbachol injections, we found an average of 299±22 Fos-positive nuclei per section within a 1x1 mm counting box on the injected side (Fig. 5) and only 11±2 in the symmetrically located box on the opposite side (p<0.001). In three animals with saline injections, there was no difference in the number of Fos-expressing cells between the two sides (51±22 per section on the injected side, and 22±9 on the opposite side). On the injected side, these counts were significantly higher in the carbachol- than saline-injected animals (p<0.001), whereas on the opposite side the difference was not significant. On the injected side, but not on the opposite side, the number of Fos-positive nuclei was positively correlated with the cumulative volume of carbachol injected (R=0.72, p<0.01; n=14) (Fig. 5C–D), whereas the correlation with the total duration of REMS-like state was not significant on either side (data not shown).
Figure 5.
Fos expression was increased in neurons of the dorsomedial pontine reticular formation near the carbachol injection site, but not in the symmetrically located region on the opposite side. The arrow in the central panel shows the center of the injection site. A: enlarged image of the 1x1 mm counting box located in the central panel on the injected side shows a very high level of Fos expression. B: paucity of Fos-positive nuclei in the symmetrically located counting box on the opposite side. C: significant correlation between the number of Fos-positive nuclei in the counting box around the injection site and the total volume of the carbachol injected in different animals. D: absence of such correlation on the side opposite to carbachol injection. Asterisks in the central panel surround a distinct group of Fos-positive cells located dorsal to the injection site. These cells expressed Fos on the injected side in all carbachol-injected animals, but not in saline-injected rats. VT - ventral tegmental nucleus; PO – nucleus pontis oralis. Thin lines in C show 95% confidence limits of the regression.
Interestingly, in all animals with carbachol injections, we found a high density of Fos-positive cells dorsal to the injection site, in the region surrounded by asterisks in the central panel of Fig. 5. Such cells were absent on the opposite side, or on either side in saline-injected animals. Since the cells were not TH-positive, their numbers were not systematically counted in this study.
Correlation of Fos expression in CA neurons with the duration of REMS-like state
Figure 6 shows examples of Fos expression in A7 neurons in two animals, one that had no REMS-like episodes (panel A, a saline-injected animal) and another that received three injections of carbachol during 140 min prior to sacrifice that triggered three relatively long REMS-episodes that had a total duration of 29 min (panel B). Characteristically, few A7 neurons contained Fos-positive nuclei in the carbachol-injected animal (only 3 present in the illustrated frame), whereas in the saline-injected animal many TH-positive cells had Fos-positive nuclei.
Figure 6.
Fos expression was reduced in A7 neurons following REMS-like episodes. A: A7 neurons in an animal that received only saline injections into the dorsomedial pons (animal 3 in Fig. 3) and had no REMS-like episodes. B: another animal that received three carbachol injections and had 29.4 min of REMS-like state during the last 140 min prior to sacrifice (animal 15 in Fig. 3). Both images show A7 neurons on the injected side. Arrows point to all TH cells that had Fos-positive nuclei. Note numerous Fos-containing TH cells in the saline-injected animal (A) and paucity of such cells in the carbachol-injected animal (B).
In the saline-injected animals, relatively many TH-positive neurons contained Fos in all CA groups. The percentage of such neurons was highest in the intermediate A1/C1 region (78.1%) and lowest in the SubC region (27.1%±1.2). Table 1 shows the mean numbers of TH-positive cells per section in each CA group. The baseline levels of Fos expression can be estimated from the intercepts of the regression lines with the Y axis for the relationships between the cumulative duration of REMS-like state and the percentage of Fos-expressing TH cells. The slopes of those regressions that were significant varied from −0.66% of Fos-positive TH cells per minute of REMS-like state for A7 neurons contralateral to the injection side to −0.37% of Fos-positive TH cells per minute of REMS-like state for SubC neurons contralateral to the injection side.
Table 1.
Regression parameters for the relationships between the percentage of Fos-expressing TH neurons and the duration and timing of REMS-like state.
| Cell group | TH cell count [mean ±SE] | Regression parameters re. cumulative duration of REMS-like state | Regression parameters re. time elapsed after last REMS-like episode | |||||
|---|---|---|---|---|---|---|---|---|
| Slope[% of Fos+TH cells/min of REMS-like state] | Y-intercept[% of Fos+TH cells for 0 time in REMS-like state] | Regression coefficient | Slope[% of Fos+TH cells/min] | Y-intercept[% of Fos+TH cells at time 0] | Regression coefficient | |||
| A7 | Ipsilateral
Contralateral Bilateral |
30.7 ± 1.8
29.7 ± 2.0 60.4 ± 3.5 |
−0.54
−0.66 −0.61 |
48.2
44.6 46.5 |
−0.56*
−0.83*** −0.73*** |
0.19
0.15 0.18 |
28.7
25.6 27.1 |
0.49 (ns)
0.52 (ns) 0.55* |
| SubC | Ipsilateral
Contralateral |
41.6 ± 2.3
45.0 ± 1.9 |
0.03
−0.37 |
28.5
28.3 |
0.06 (ns)
−0.53* |
0.11
0.14 |
24.8
15.9 |
0.55*
0.40 (ns) |
| A5-rostral | ||||||||
| Ipsilateral
Contralateral Bilateral |
53.4 ± 4.2
48.0 ± 3.4 101 ± 6.4 |
−0.49
−0.50 −0.48 |
47.2
51.0 48.8 |
−0.56*
−0.66** −0.69** |
0.17
0.26 0.21 |
33.3
31.9 32.9 |
0.36 (ns)
0.64* 0.56* |
|
| A5-caudal | ||||||||
| Ipsilateral
Contralateral Bilateral |
23.3 ± 2.7
16.9 ± 3.2 50.2 ± 5.5 |
−0.33
−0.43 −0.39 |
42.6
39.3 41.3 |
−0.30 (ns)
−0.36 (ns) −0.38 (ns) |
0.11
0.05 0.08 |
32.9
29.4 31.6 |
0.18 (ns)
0.09 (ns) 0.14 (ns) |
|
| A2/C2 | Bilateral | 182 ± 8.7 | −0.28 | 38.8 | −0.36 (ns) | 0.37 | 20.1 | 0.82** |
| A1 | Ipsilateral
Contralateral Bilateral |
47.8 ± 4.8
40.5 ± 3.9 88.4 ± 6.5 |
−0.54
0.03 −0.33 |
75.6
68.2 72.6 |
−0.42 (ns)
0.02 (ns) −0.26 (ns) |
0.41
0.14 0.30 |
48.9
63.4 54.5 |
0.56 (ns)
0.17 (ns) 0.41 (ns) |
| A1/C1 (inter- med) | Ipsilateral
Contralateral Bilateral |
61.0 ± 4.1
59.1 ± 5.7 120 ± 8.2 |
−0.04
0.11 0.02 |
86.8
86.8 86.8 |
−0.06 (ns)
0.18 (ns) 0.03 (ns) |
0.08
0.02 0.06 |
83.5
88.5 85.4 |
0.22 (ns)
0.06 (ns) 0.17 (ns) |
| C1 | Ipsilateral
Contralateral Bilateral |
166 ± 12
159 ± 8.3 326 ± 20 |
−0.15
−0.22 −0.18 |
64.9
63.8 64.1 |
−0.20 (ns)
−0.39 (ns) −0.31 (ns) |
−0.08
0.11 0.02 |
66.9
56.4 61.3 |
−0.19 (ns)
0.39 (ns) 0.06 (ns) |
The second column from the left represents the total counts of TH-positive neurons within each CA group obtained from every 5th 35 μm thick section. The next three columns show linear regression parameters (slope, Y-intercept, regression coefficient) for the relationship between the percentage of Fos+TH cells and the cumulative duration of REMS-like episodes during 140 min prior to sacrifice. The Y-intercept is the intersection of the regression line with the Y axis at time 0, i.e., no time spent in REMS-like state prior to sacrifice. The right section shows the corresponding linear regression parameters for the relationship between the percentage of Fos+TH cells and the interval between the last REMS-like episode and the time of sacrifice. In this case, the slopes are positive and time 0 represents the time right after the last REMS-like episode. Significance levels for linear regression coefficients:
P<0.05,
P<0.01,
P<0.001, ns - not significant.
The percentage of TH-positive neurons with Fos-stained nuclei was negatively correlated with the total duration of REMS-like state in the rostral A5 (Table 1) and A7 regions (Fig. 7). In these regions, the strength of the correlation (regression coefficient) was similar ipsi- and contralaterally to the carbachol injection site (Table 1). In the SubC region, the correlation was significant on the side opposite to carbachol injection (Fig. 8A) but it was absent on the carbachol-injected side (Fig. 8B). In the A2/C2, C1 and caudal A5 regions, there were trends for reduced Fos expression with increasing duration of REMS-like state, but the correlations did not reach statistical significance (Table 1). In the A1 and intermediate A1/C1 regions, such trends were even weaker or absent.
Figure 7.
The percentage of A7 neurons expressing Fos was negatively correlated with the cumulative duration of REMS-like state during the last 140 min prior to sacrifice. The regressions were statistically significant for both the carbachol/saline-injected (A) and opposite (B) sides. Open circles – data from the three animals that received saline injections only; filled circles – data from the 14 carbachol-injected animals. Thin lines show 95% confidence limits of the regressions.
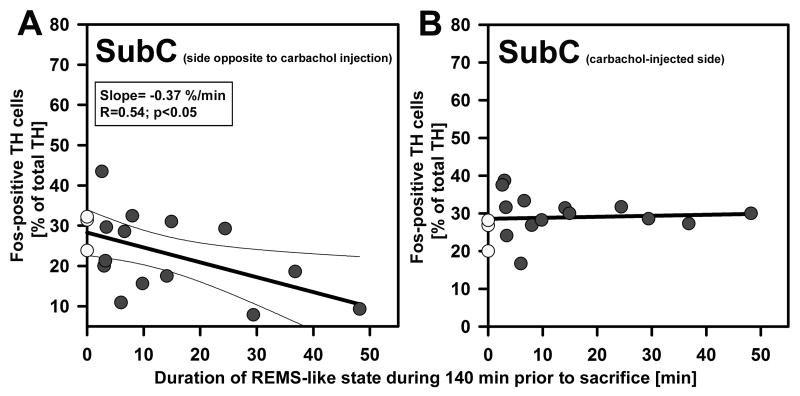
Figure 8.
The correlation between Fos expression and duration of REMS-like state during the last 140 min prior to sacrifice was significant for SubC neurons located on the side opposite to carbachol injections (A), but such a correlation was absent on the carbachol-injected side (B). The difference between the two sides is ascribed to the relative proximity of the SubC region to the injection site and excitatory effects of carbachol on TH-positive SubC neurons that could mask the Fos-reducing effect of REMS-like state on the injected side. Open circles – data from the three animals that received saline injections only; filled circles – data from the 14 carbachol-injected animals. Thin lines in A show 95% confidence limits of the regression.
Correlation of Fos expression in CA neurons with the duration of the period between the last REMS-like episode and sacrifice
The analysis described in the preceding section revealed that REMS-like episodes were associated with decreased Fos expression in TH neurons in some CA groups. This suggested that Fos expression in TH cells decreased during and/or right after each REMS-like episode and then increased during the baseline periods that separated successive episodes. To verify this prediction, we conducted regression analysis between Fos expression in CA neurons and the time elapsed from the last REMS-like episode to the time of animal sacrifice. This analysis revealed significant positive correlations for SubC (on the injected side), rostral A5 (Table 1), A7 (Fig. 9A) and A2/C2 (Fig. 9B) regions.
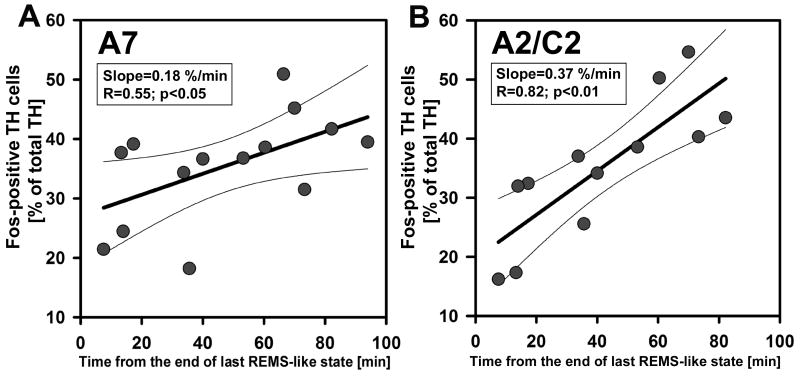
Figure 9.
A7 (A) and A2/C2 (B) neurons had significant positive correlations between the time elapsed from the last REMS-like episode to the time of animal’s sacrifice and the percentage of TH cells expressing Fos, suggesting that Fos levels were reduced during, or soon after, each REMS-like episode and then gradually recovered. Thin lines show 95% confidence limits of the regressions.
Absence of correlation between Fos expression in CA neurons and the dose of carbachol or arterial blood pressure
One potential confounder present in our experimental design was that the animals with higher total amount of REMS-like state tended to have received larger doses of carbachol. However, the relationship was only partial because there were carbachol injections that did not trigger REMS-like episodes and there were also spontaneous REMS-like episodes that were not directly preceded by carbachol injections (Fig. 3). We took advantage of this dissociation to assess whether there was a correlation between Fos expression in TH-positive neurons and the total dose of carbachol injected in each experiment. Linear regression analysis of this relationship yielded no significant correlation for any of the studied neuronal group. Thus, in contrast to the results with either the cumulative duration of REMS-like state or the period between the last REMS-like episode and the time of sacrifice, it did not appear that the dose of carbachol injected into the pons determined the level of Fos expression in pontomedullary TH neurons.
Since REMS-like responses are often accompanied in urethane-anesthetized rats by ~6 mmHg increases of arterial blood pressure (Fenik et al., 2005) and it was reported that experimentally-induced hypotension increased Fos expression in some CA neurons (Chan and Sawchenko, 1994; Graham et al., 1995; Dampney et al., 2003), we also tested whether the percentage of cells expressing Fos was correlated with the mean level of systolic blood pressure measured in different animals at 120, 80 or 40 min before the perfusion. However, as with the analysis in relation to the dose of carbachol, this correlation was not significant.
DISCUSSION
We found that Fos expression was reduced proportionally to the amount of REMS-like state in A7, SubC and rostral A5 neurons, and in A2/C2 neurons it increased with the time between the last REMS-like episode and the time of sacrifice. In contrast, in A1/C1 neurons, Fos levels were not correlated with either the total amount REMS-like state or the time elapsed after the last episode. We determined that the presence and amount of REMS-like state produced during about 2 h (140 min) prior to animal’s sacrifice had a higher impact on Fos levels in pontomedullary TH neurons than any longer periods, and that there was no correlation between the amount of carbachol injected into the pons and Fos expression in any CA group. Considering that, in most central neurons, Fos expression increases with the level of activity, these results are consistent with prior evidence that SubC and A5 cells cease firing during REM sleep (Reiner, 1986; Fenik et al., 2002) and suggest, for the first time, that pontine A7 and medullary A2/C2 neurons also have reduced activity during REMS, whereas A1/C1 cells do not have this feature.
Technical considerations
In unanesthetized behaving animals, few pontomedullary CA cells have Fos-stained nuclei under the baseline conditions (Chan and Sawchenko, 1994; Yamuy et al., 1998; Maloney et al., 1999; Dampney et al., 2003). In contrast, the percentage of TH-positive cells expressing Fos in urethane-anesthetized, vagotomized, paralyzed and artificially ventilated rats under the baseline conditions of our study (over 5 h of recording with saline microinjections into the pontine reticular formation) was relatively high, ranging from about 27% in SubC neurons to 78% in A1/C1 neurons. Two factors may be considered as contributing to elevated baseline Fos levels in TH cells in our experiments. One is CO2-induced elevation of c-fos expression in CA neurons (Haxhiu et al., 1996). In our study on anesthetized animals artificially ventilated with oxygen-enriched air, a relatively high CO2 level (5.5–6.0%) as compared to intact animals was needed to maintain steady respiratory modulation of XII nerve activity. However, since ventilation was kept constant throughout the experimental protocol, there were no changes in CO2 level in relation to REMS-like episodes. On the other hand, an elevated baseline level of Fos expression in our preparation, whether caused by CO2 or other factors, technically could help detect decrements in Fos expression. Of note here is that, of the two previous attempts to investigate the relationship between Fos expression in pontine NE cells (LC and SubC) and the amount of natural or carbachol-induced REMS, reduced Fos levels was detected in one (Maloney et al., 1999) but not in the other study (Yamuy et al., 1998).
Another factor that may influence Fos expression in CA neurons is the level of arterial blood pressure. Experimentally induced, profound hypotension increased Fos expression in A1/C1 and A5 neurons (Chan and Sawchenko, 1994; Graham et al., 1995; Dampney et al., 2003). In our anesthetized animals, arterial blood pressure was 98±4.8 mmHg, thus lower than in behaving animals, but within physiologic limits, and nowhere near the blood pressure nadirs produced for prolonged periods in those other studies. Since REMS-like episodes are accompanied by arterial blood pressure increases (Fenik et al., 2005), there could be a concern that transient blood pressure increases contributed to the correlation with REMS-like episodes that are found in this study. However, these transient changes averaged only 6 mmHg (Fenik et al., 2005), and we found no correlation between the level of blood pressure in individual animals and the level of Fos expression in CA cell groups. This included A1/C1 cells in which Fos expression was found to be exquisitely sensitive to hypotensive challenges (Chan and Sawchenko, 1994) but was not significantly correlated with the duration of REMS-like state in our study. Thus, it is unlikely that the mean level or changes of arterial blood pressure significantly contributed to the changes in Fos expression in our experiments.
Since the level of Fos expression generally correlates with the level of neuronal activity (Dragunow and Faull, 1989; Morgan and Curran, 1991), the relatively high levels of Fos expression in TH cells in our study could be taken to suggest that the baseline levels of activity in CA neurons in urethane-anesthetized rats was higher than in intact animals. However, our recordings from LC (Kubin, 2001) or A5 (Fenik et al., 2002) neurons in anesthetized, paralyzed and artificially ventilated rats revealed mean firing rates of the order of 1–2 Hz, thus slightly lower than those typically encountered in LC cells in chronically instrumented rats during quiet wakefulness (Aston-Jones and Bloom, 1981; Gervasoni et al., 1998). Therefore, it appears that the baseline level of transcriptional and/or metabolic activity is elevated in CA cells in urethane-anesthetized rats without a concomitant increase of their baseline firing rates.
NE neurons of the LC and SubC region cease firing during natural REMS (Aston-Jones and Bloom, 1981; Reiner, 1986) and both LC and A5 neurons are silenced during REMS-like episodes elicited by pontine carbachol in anesthetized rats (Kubin, 2001; Fenik et al., 2002). Accordingly, we expected a decrease of Fos expression in some CA cells following a period of repeated occurrence of REMS-like episodes. In our study, anesthesia minimized potentially confounding effects of behavior and environmental conditions which may alter the outcomes of such experiments, as suggested by contradictory results from two studies in which Fos expression was assessed in pontine cholinergic neurons (Maloney et al., 1999; Verret et al., 2005). Compared to behaving animals, the carbachol-injected anesthetized rat is a simplified model of REMS, but nevertheless it exhibits major hallmarks of this state, including cortical activation, theta-like activity in the hippocampus, silencing of LC and A5 cells and depression of activity in XII motoneurons (Kubin, 2001; Fenik et al., 2002; Kubin and Fenik, 2004; Fenik et al., 2005). In addition, in both chronically instrumented, behaving rats (Alam et al., 2005) and anesthetized rats (Lu et al., 2007), the occurrence of REMS, or REMS-like state, is blocked by activation of neurons located in a well-established wake-promoting region of the posterior hypothalamus. The anesthetized rat carbachol model does not generate phasic events of REMS, such as eye movements, bursts of motoneuronal activity or rapid variations of respiratory rate and arterial blood pressure. For the purpose of the present study, the absence of those factors could minimize the inter-individual variability and facilitate detection of the association between the REMS-like state and Fos expression.
Our initial linear regression analysis of the correlations between Fos expression in CA neurons and the duration of REMS-like state indicated that REMS-like episodes generated during the last 140 min prior to sacrifice had a higher impact on decrements in Fos expression in TH-positive cells than any longer time periods. Based on this finding, we conducted our analysis over a period of 140 min. This is similar to the periods used in prior studies (1–3 h) that correlated natural behavioral states with Fos expression in the brainstem (e.g. Merchant-Nancy et al., 1995; Maloney et al., 1999; Verret et al., 2005). Notably, the highest percentage of REMS during the analyzed periods was 50% or less in those earlier studies and 34% in our study, and yet significant correlations were detected. This suggests that relatively brief but repeatedly occurring periods of natural REMS or REMS-like state reduce Fos levels powerfully enough so that the effect cannot be readily “erased” during other states that occur between REMS episodes and often last longer than the periods of REMS. Accordingly, the presence of significant negative correlations between Fos expression in TH-positive neurons and the amount of REMS (Maloney et al., 1999), or REMS-like state (this study), suggests that the degradation of Fos associated with REMS-like episodes either occurs more rapidly than its subsequent re-expression between the episodes or that Fos-degrading effect of REMS considerably outlasts the duration of each REMS-like episode. Without one or both of these conditions fulfilled, one would not be able to detect decrements in Fos expression following multiple REMS-like episodes that occupy less than 50% of time prior to sacrifice.
Fos expression in neuronal nuclei is estimated to occur over a period of 30 min to 2 h after an activating stimulus (Dragunow and Faull, 1989; Morgan and Curran, 1991; Zangenehpour and Chaudhuri, 2002). Data from the primary visual cortex suggest that c-fos mRNA expression occurs within the first 30 min of this period but little Fos protein is produced (Zangenehpour and Chaudhuri, 2002). However, Fos protein entirely disappears from neuronal nuclei in the cortex within 1 h after transition from wakefulness to sleep (Basheer et al., 1997). Together, these data suggest that the rate of Fos degradation or dissipation is higher than its rate of re-expression. In the absence of specific information about these rates in CA neurons, as in previous studies (Basheer et al., 1997; Maloney et al., 1999; Verret et al., 2005), our analysis assumed linear changes of Fos expression with the duration of the inducing stimulus or state. Significant correlations that we detected suggest that the assumption, albeit simplistic, was reasonably fulfilled in our experimental conditions.
As noted above, different CA cell groups had different baseline levels of Fos expression. In addition, our estimates of the rate of Fos expression increase after the last REMS-like episode were 1.8–2.1% of new cells with Fos-positive nuclei per 10 min for A5 and A7 neurons and nearly twice that (3.7% of new cells with Fos-positive nuclei per 10 min) for A2/C2 neurons (Table 1). Similarly, among the cell groups that had statistically significant correlations with the total duration of REMS-like state, the regression slopes varied from 3.7% of cells loosing Fos per 10 min of REMS-like state in SubC neurons to 6.6% of cells for A7 neurons (see slopes in Table 1). These estimates suggest that there are substantial differences in the rate of Fos accumulation and decay among different groups of pontomedullary TH-positive neurons and, importantly, it indeed appears that Fos degradation associated with REMS-like episodes is more rapid than Fos re-expression that presumably follows each REMS-like episode.
Fos expression at the carbachol injection site
Carbachol is a mixed muscarinic-nicotinic receptor agonist. In vitro studies in slices show that similar proportions of cells located in the dorsomedial pontine tegmentum are excited and inhibited by bath application of carbachol (Leonard and Llinás, 1994; Brown et al., 2006). Microinjections of carbachol into the dorsal pontine tegmentum in vivo also excite some and inhibit other neurons located near the injection site (Shiromani and McGinty, 1986; El Mansari et al., 1990). In chronically instrumented, behaving cats, dorsal pontine injections of carbachol at doses and volumes much larger than those used in the present study (2–4 μg in 250 nl vs. 37 ng in 20 nl in our study) produced massive Fos expression around the injection site (Yamuy et al., 1993; Shiromani et al., 1996). In one such study, consistent with extensive evidence that REMS-like state is effectively elicited by carbachol microinjections into the dorsomedial pontine tegmentum (reviewed by Kubin, 2001), the total number of Fos-expressing pontine reticular cells was positively correlated with the amount of REMS-like state (Shiromani et al., 1995). We also found large numbers of Fos-expressing cells near the injection site despite our use of much lower doses of carbachol. However, the counts of Fos-positive nuclei at the injection site were correlated only with the amount of injected carbachol but not with the duration of REMS-like state. This suggests that the dorsomedial pontine region where carbachol triggers REMS-like episodes contains many neurons excited by carbachol but only a subset of those belong to the REMS-triggering network.
We found unilateral expression of Fos in a distinct group located dorsal to the injection site in carbachol- but not saline-injected animals. These cells may correspond to those expressing Fos following sleep rebound after REMS deprivation that were previously described by Maloney et al., (1999) and Verret et al., (2005) at the junction between the laterodorsal tegmental nucleus and ventrolateral periaqueductal gray (cf. the cross-section at AP-8.60 in Fig. 1 of Verret et al., 2005). This location overlaps with both cholinergic neurons of the laterodorsal tegmental nucleus and GABA-ergic neurons located just lateral to this nucleus (Maloney et al., 1999; Verret et al., 2005). By double-labeling for neuronal nitric oxide synthase and Fos in a subset of our sections, we determined that the cells in our study were not cholinergic (Rukhadze and Kubin, unpublished observations), but it remains to be determined whether some of them are GABA-ergic.
Fos expression in A5 and SubC neurons following REMS-like state in urethane-anesthetized rats
NE neurons of the A5 and SubC regions cease firing during natural REMS or REMS-like state elicited by carbachol in anesthetized rats (Reiner, 1986; Fenik et al., 2002). Our finding of a significant negative correlation between Fos expression in these neurons and the duration of REMS-like state suggests that Fos expression can provide insight into the changes in CA cell activity associated with REMS.
The relationship between Fos expression and duration of REMS-like state in SubC neurons was present on the side opposite to carbachol injection but not on the carbachol-injected side. However, we found significant positive correlation between Fos expression in SubC NE neurons on the injected side and the time elapsed after the last REMS episode. Together, these data show that the primary effect of REMS-like state on SubC neurons is to reduce their Fos expression. The lack of correlation with cumulative duration of REMS-like state on the injected side could be caused by an excitatory effect of carbachol on SubC neurons that masked the Fos-reducing effects of REMS-like state. This interpretation is supported by reports that cholinergic agonists excite NE neurons (Engberg and Svensson, 1980; Koyama and Kayama, 1993). Thus, for the injected side, the discrepancy between significant correlation with time after the last REMS-like episode and the absence of significant correlation with cumulative duration of REMS-like state could be due to a gradual desensitization of the excitatory effects of carbachol at the injection site.
We found significant negative correlation between the total duration of REMS-like state and Fos expression in TH-positive neurons located in the rostral, but not caudal, portion of the A5 group. Neurons located in both parts of the A5 group have projections to the intermediolateral cell column of the spinal cord, nucleus of the solitary tract and other brainstem nuclei (Loewy et al., 1986; Fritschy and Grzanna, 1990; Clark and Proudfit, 1993; Rukhadze and Kubin, 2007), but only rostral A5 neurons have ascending projections to the hypothalamus and basal forebrain (Byrum and Guyenet, 1987). Rostral and caudal A5 neurons also differentially express various CA markers; caudal A5 neurons stain relatively weakly for TH, but have high levels of dopamine-beta-hydroxylase (DBH), whereas rostral A5 cells are strongly positive for both TH and DBH (Goodchild et al., 2001). These differences prompted us to separately analyze the two regions, a distinction also used by others (Lyons and Grzanna, 1988; Clark and Proudfit, 1993). The trend towards decreased Fos levels following REMS-like state in caudal A5 neurons suggests that the caudal A5 neurons also decrease their activity during REMS-like state. The relatively weaker regression indices found for the caudal A5 region suggest a smaller magnitude of this effect in that part of A5 than in the rostral region.
Different effects of REMS-like state on Fos expression in different pontomedullary CA cell groups
A7 neurons had the strongest negative correlation between the total duration of REMS-like state and the percentage of Fos-positive neurons. They also had a strong positive correlation between the time elapsed after last REMS-like episode and Fos expression (Table 1). The decreased Fos expression in A7 NE neurons in association with REMS-like state provides the first evidence that these neurons may have reduced or abolished activity during REMS, as it is typical of LC (Aston-Jones and Bloom, 1981), SubC (Reiner, 1986) and at least some A5 neurons (Fenik et al., 2002).
The results with A2/C2 neurons were less unequivocal than those with A7 neurons. There was only a trend for negative correlation between the total duration of REMS-like state and percentage of Fos-positive neurons, but the positive correlation with the time elapsed after the last REMS-like episode was highly significant. The regression indices suggested that A2/C2 cells had particularly fast time-course of Fos re-expression after REMS-like episodes (they had the steepest slope of Fos recovery among all groups that yielded significant correlations – Table 1). As discussed earlier, this could make detection of the effects of repeated REMS-like episodes on Fos expression difficult, whereas Fos recovery after the last episode could still be detected.
In contrast to pontine NE neurons and medullary A2/C2 neurons, Fos expression in the medullary A1/C1 cells was not significantly correlated with either the total duration of REMS-like state or the time elapsed after last REMS-like episode. Since the A1/C1 region contains functionally and neurochemically distinct populations of CA neurons that also have different projections (Hökfelt et al., 1984; Tucker et al., 1987; Sakai et al., 1990; Card et al., 2006), we divided the region into three sub-regions. The numbers of Fos-positive TH neurons in A1, intermediate A1/C1 and C1 regions declined slightly in association with the duration of REMS-like state, but none of those correlations was significant, nor was any of the correlations with the time elapsed after the last REMS-like episode. Compared to the results with A7 and A2/C2 neurons, this suggests that A1/C1 neurons do not have reduced activity during REMS.
Functional implications
Pontomedullary CA neurons control sensory transmission, motor activity and cardiorespiratory outputs. Accordingly, the decrements or abolition of activity in some pontomedullary CA neuronal groups during REMS suggested by our results may alter neural transmission in multiple and functionally diverse central pathways in a state-dependent manner. For example, NE has been suggested to enhance the gain of transmission in somatosensory pathways (Morilak and Jacobs, 1985; Stafford and Jacobs, 1990). Spinally projecting A7 neurons may be an important component of the descending neuronal system that modulates nociception (Clark and Proudfit, 1991). Neurons of the A5, A7, SubC and LC regions have spinal projections that terminate directly on motoneurons (Byrum and Guyenet, 1987; Fritschy and Grzanna, 1990; Clark and Proudfit, 1991; Clark and Proudfit, 1993). A5, A7 and SubC neurons are also the main source of NE innervation of the medullary XII motor nucleus (Rukhadze and Kubin, 2007). In XII motoneurons, withdrawal of excitation mediated by NE is a major cause of REMS-related depression of their activity (Fenik et al., 2005).
According to the classic concepts of the generation of REMS, reduced or abolished activity in NE neurons is required for the occurrence of the state (McCarley and Hobson, 1975). Although LC traditionally has been seen as a key nucleus responsible for this REMS-permitting effect, the contributions of other NE groups have not been assessed. In anesthetized rats, microinjections into the A5 region of the α2-adrenergic agonist, clonidine, which inhibits NE neurons, triggered REMS-like episodes like those evoked from the dorsomedial pons by carbachol (Fenik et al., 2002). This suggests that activity of some A5 neurons have a REMS-opposing action. Data also suggest that some neurons located in the medullary nucleus of the solitary tract have sleep promoting effects (Puizillout, 1986). Our finding that some A2/C2 neurons have reduced Fos expression following REMS-like episodes combined with anatomical evidence that some A2/C2 neurons project to the forebrain regions involved in the regulation of sleep (Blessing et al., 1982; Sakai et al., 1990; Reyes and Van Bockstaele, 2006) lends further support to this suggestion. Similarly, some LC, A5, A7 and SubC neurons project to both wake-promoting regions of the posterior hypothalamus and sleep-promoting regions of the anterior hypothalamus (Olson and Fuxe, 1972; Aston-Jones et al., 1986; Chou et al., 2002). Thus, projections from multiple CA neuronal groups that have state-dependent activity may contribute to the regulation of sleep.
A1/C1, A2/C2 and A5 neurons play important roles in cardiovascular and neuroendocrine regulation (Chan and Sawchenko, 1994; Graham et al., 1995; Schreihofer and Guyenet, 2000). Many of them project to the hypothalamic paraventricular nucleus or the thoracic spinal cord (Ross et al., 1985; Tucker et al., 1987; Schreihofer and Guyenet, 2000; Card et al., 2006), where they regulate fluid balance and sympathetic output, respectively (reviewed by Guyenet, 2006). Of these three groups, the A1/C1 group is most numerous and the only one for which our data did not suggest decrements of activity in association with REMS. Such “cardiovascular” A1/C1 neurons are well suited to maintain stable mean arterial blood pressure during REMS despite all the rapid perturbations characteristic of this state that probably originate outside the A1/C1 group.
SUMMARY
We found that all pontomedullary CA neurons except those of the A1/C1 group have reduced Fos expression in relation to REMS-like state elicited by carbachol, or at least exhibit a trend in this direction (caudal A5 neurons). For SubC and A5 neurons, this finding can be related to prior evidence that they cease firing during REMS. Accordingly, our data with A7 and A2/C2 neurons, the activity of which has not been previously recorded during REMS, suggest that these cells also have reduced activity during this state. If so, it appears that a reduction or abolition of activity during REMS is a common feature of most pontomedullary CA neurons, with the A1/C1 group being a notable exception from this rule.
Acknowledgments
The study was supported by a National Institutes of Health grant HL-47600. We thank Ms. Tyana Singletary, Ms. Janet Lee and Ms. Sandra Song for technical assistance.
Abbreviations
- CA
catecholaminergic
- DBH
dopamine-beta-hydroxylase
- EEG
electroencephalogram
- GABA
gamma-amino butyric acid
- LC
locus coeruleus
- NE
noradrenergic
- PO
nucleus pontis oralis
- REMS
rapid eye movement sleep
- scp
superior cerebellar peduncle
- SE
standard error
- SubC
sub-coeruleus
- TH
tyrosine hydroxylase
- VT
ventral tegmental nucleus
- XII
hypoglossal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005:563, 2–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Ross CA, Pickel VM, Joh TH, Reis DJ. Distribution of dopamine-, noradrenaline-, and adrenaline- containing cell bodies in the rat medulla oblongata: demonstrated by the immunocytochemical localization of catecholamine biosynthetic enzymes. J Comp Neurol. 1982:212, 173–187. doi: 10.1002/cne.902120207. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981:1, 876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickel WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986:234, 734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Basheer R, Sherin JE, Saper CB, Morgan JI, McCarley RW, Shiromani PJ. Effects of sleep and wake-induced c-fos expression. J Neurosci. 1997:17, 9746–9750. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Jaeger CB, Ruggiero DA, Reis DJ. Hypothalamic projections of medullary catecholamine neurons in the rabbit: A combined catecholamine fluorescence and HRP transport study. Brain Res Bull. 1982:9, 279–286. doi: 10.1016/0361-9230(82)90141-1. [DOI] [PubMed] [Google Scholar]
- Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006:143, 739–755. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrum CE, Guyenet PG. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol. 1987:261, 529–542. doi: 10.1002/cne.902610406. [DOI] [PubMed] [Google Scholar]
- Card PJ, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J Comp Neurol. 2006:499, 840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol. 1994:348, 433–460. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006:174, 1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammel T, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002:22, 977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Sleep deprivation and c-fos expression in the rat brain. J Sleep Res. 1995:4, 92–106. doi: 10.1111/j.1365-2869.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000:885, 303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 1991:547, 279–288. doi: 10.1016/0006-8993(91)90972-x. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projections of noradrenergic neurons in the A5 catecholamine cell group to the spinal cord in the rat: anatomical evidence that A5 neurons modulate nociception. Brain Res. 1993:616, 200–210. doi: 10.1016/0006-8993(93)90210-e. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cel Mol Neurobiol. 2003:23, 597–616. doi: 10.1023/A:1025080314925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Met. 1989:29, 261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sakai K, Jouvet M. Responses of presumed cholinergic mesopontine tegmental neurons to carbachol microinjections in freely moving cats. Exp Brain Res. 1990:83, 115–123. doi: 10.1007/BF00232199. [DOI] [PubMed] [Google Scholar]
- Engberg G, Svensson TH. Pharmacological analysis of a cholinergic receptor mediated regulation of brain norepinephrine neurons. J Neural Transm. 1980:49, 137–150. doi: 10.1007/BF01245220. [DOI] [PubMed] [Google Scholar]
- Fenik V, Fenik P, Kubin L. A simple cuff electrode for nerve recording and stimulation in acute experiments on small animals. J Neurosci Meth. 2001:116, 147–150. doi: 10.1016/s0165-0270(01)00340-5. [DOI] [PubMed] [Google Scholar]
- Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002:93, 1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. (REM sleep-like atonia of hypoglossal XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005:172, 1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Demonstration of two separate descending pathways to the rat spinal cord:evidence for an intragriseal trajectory of locus coeruleus axons in the superficial layers of the dorsal horn. J Comp Neurol. 1990:291, 552–582. doi: 10.1002/cne.902910406. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited bt GABA during sleep. Eur J Neurosci. 1998:10, 964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Phillips JK, Lipski J, Pilowsky PM. Differential expression of catecholamine synthetic enzymes in the caudal ventral pons. J Comp Neurol. 2001:438, 457–467. doi: 10.1002/cne.1328. [DOI] [PubMed] [Google Scholar]
- Graham JC, Hoffman GE, Sved AF. C-fos expression in brain in response to hypotension and hypertension in conscious rats. J Auton Nerv Syst. 1995:55, 92–104. doi: 10.1016/0165-1838(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006:7, 335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006:26, 3037–3044. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol. 1996:105, 35–45. doi: 10.1016/0034-5687(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Johansson O, Goldstein M. Central catecholamine neurons as revealed by immunohistochemistry with special reference to adrenaline neurons. In: Björkland A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy, Classical Transmitters in the CNS. Part I. Vol. 2. Elsevier; Amsterdam: 1984. pp. 157–276. [Google Scholar]
- Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch Ital Biol. 2004:142, 379–396. [PubMed] [Google Scholar]
- Kalia M, Woodward DJ, Smith WK, Fuxe K. Rat medulla oblongata. IV. Topographical distribution of catecholaminergic neurons with quantitative three-dimensional computer reconstruction. J Comp Neurol. 1985c:233, 350–364. doi: 10.1002/cne.902330305. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Kayama Y. Mutual interactions among cholinergic, noradrenergic and serotonergic neurons studied by ionophoresis of these transmitters in rat brainstem nuclei. Neuroscience. 1993:55, 1117–1126. doi: 10.1016/0306-4522(93)90325-a. [DOI] [PubMed] [Google Scholar]
- Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001:139, 147–168. [PubMed] [Google Scholar]
- Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol. 2004:143, 235–249. doi: 10.1016/j.resp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001:21, 7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CS, Llinás R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994:59, 309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Marson L, Parkinson D, Perry MA, Sawyer WB. Descending noradrenergic pathways involved in the A5 depressor response. Brain Res. 1986:386, 313–324. doi: 10.1016/0006-8993(86)90168-x. [DOI] [PubMed] [Google Scholar]
- Lu JW, Fenik VB, Branconi JL, Mann GL, Rukhadze I, Kubin L. Disinhibition of perifornical hypothalamic neurones activates noradrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats. J Physiol. 2007:582, 553–67. doi: 10.1113/jphysiol.2007.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Grzanna R. Noradrenergic neurons with divergent projections to the motor trigeminal nucleus and the spinal cord: a double retrograde labeling study. Neuroscience. 1988:26, 681–693. doi: 10.1016/0306-4522(88)90174-1. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999:19, 3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975:189, 58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- Merchant-Nancy H, Vazquez J, Garcia F, Drucker-Colin R. Brain distribution of c-fos expression as a result of prolonged rapid eye movement (REM) sleep period duration. Brain Res. 1995:681, 15–22. doi: 10.1016/0006-8993(95)00275-u. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Ann Rev Neurosci. 1991:14, 421–51. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Jacobs BL. Noradrenergic modulation of sensorimotor processes in intact rats: the masseteric reflex as a model system. J Neurosci. 1985:5, 1300–1306. doi: 10.1523/JNEUROSCI.05-05-01300.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L, Fuxe K. Further mapping out of central noradrenaline neuron systems: projections of the ‘subcoeruleus’ area. Brain Res. 1972:43, 289–295. doi: 10.1016/0006-8993(72)90299-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Compact. 3. Academic Press; San Diego: 1997. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Puizillout JJ. Nucleus tractus solitarius, serotonin and regulation of vigilance. Rev Electroencephal Neurophysiol Clin. 1986:16, 95–106. doi: 10.1016/s0370-4475(86)80001-6. [French] [DOI] [PubMed] [Google Scholar]
- Reiner PB. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Brain Res. 1986:378, 86–96. doi: 10.1016/0006-8993(86)90288-x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Van Bockstaele EJ. Divergent projections of catecholaminergic neurons in the nucleus of the solitary tract to limbic forebrain and medullary autonomic brain regions. Brain Res. 2006:1117, 69–79. doi: 10.1016/j.brainres.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol. 1985:242, 511–534. doi: 10.1002/cne.902420405. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat. 2007:33, 23–33. doi: 10.1016/j.jchemneu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Fenik VB, Branconi JL, Kubin L. Fos expression in pontine noradrenergic cell groups negatively correlates with the duration of carbachol-induced REM sleep-like state in urethane-anesthetized rats. Sleep. 2007;30(Suppl):A19. [Google Scholar]
- Sakai K, Yoshimoto Y, Luppi PH, Fort P, El Mansari M, Salvert D, Jouvet M. Lower brainstem afferents to the cat posterior hypothalamus: a double-labeling study. Brain Res Bull. 1990:24, 437–55. doi: 10.1016/0361-9230(90)90098-k. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996:271, 216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, McGinty DJ. Pontine neuronal response to local cholinergic infusion: relation to REM sleep. Brain Res. 1986:386, 20–31. doi: 10.1016/0006-8993(86)90137-x. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Malik M, Winston S, McCarley RW. Time course of Fos-like immunoreactivity associated with cholinergically induced REM sleep. J Neurosci. 1995:15, 3500–3508. doi: 10.1523/JNEUROSCI.15-05-03500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani PJ, Winston S, McCarley RW. Pontine cholinergic neurons show Fos-like immunoreactivity associated with cholinergically induced REM sleep. Mol Brain Res. 1996:38, 77–84. doi: 10.1016/0169-328x(95)00325-m. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DH-saporin. Am J Physiol. 2000:279, 729–742. doi: 10.1152/ajpregu.2000.279.2.R729. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Brainstem mechanisms generating REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 2. Philadelphia: Saunders; 1994. pp. 125–144. [Google Scholar]
- Stafford IL, Jacobs BL. Noradrenergic modulation of the masseteric reflex in behaving cats. I. Pharmacological studies. J Neurosci. 1990:10, 91–98. doi: 10.1523/JNEUROSCI.10-01-00091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. J Comp Neurol. 1987:259, 591–603. doi: 10.1002/cne.902590408. [DOI] [PubMed] [Google Scholar]
- Verret L, Leger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci. 2005:21, 2488–2504. doi: 10.1111/j.1460-9568.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Mancillas JR, Morales FR, Chase MH. C-fos expression in the pons and medulla of the cat during carbachol-induced active sleep. J Neurosci. 1993:13, 2703–2718. doi: 10.1523/JNEUROSCI.13-06-02703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamuy J, Sampogna S, Morales FR, Chase MH. C-fos expression in mesopontine noradrenergic and cholinergic neurons of the cat during carbachol-induced active sleep: a double-labeling study. Sleep Res Online. 1998:1, 28–40. [PubMed] [Google Scholar]
- Zangenehpour S, Chaudhuri A. Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Mol Brain Res. 2002:109, 221–225. doi: 10.1016/s0169-328x(02)00556-9. [DOI] [PubMed] [Google Scholar]