Abstract
In this report, we use single-molecule spectroscopic method to study emission behaviors of streptavidin conjugated quantum dots immobilized on biotinylated-BSA (bovine serum albumin) monolayer near non-continuous rough silver nanostructure. We observed greatly reduced blinking and enhanced emission fluorescence of quantum dots next to silver island films.
Keywords: Single-molecule spectroscopy, quantum dot, silver island films, blinking
Introduction
Semiconducting quantum dots (QDs) have been reported to posses numerous photophysical properties that are superior to those of organic fluorophores. The emission properties of quantum dots, especially high-absorption cross section, exceptional photostability, wide excitation spectra and narrow emission bands, are important to live cell imaging(1-3) and FRET biosensors(4). However, single Quantum Dot shows strong fluorescence blinking. The fluorescence from individual QDs turns “on” and “off” on timescales ranging from milliseconds to many minutes. The long “off” periods are the main restriction for the use of QDots in imaging applications and limits their applicability in single molecule detection. The origin of the fluorescence intermittency has been studied in detail.(5-7) It has been postulated that blinking arises from random ionization events that eject a carrier from the QD. The distribution of “on”- and “off”-time differs from the exponential or near-exponential distributions observed in most other types of single fluorophores.
Many previous studies on blinking of the bright fraction and on dark fractions have been based on measurements of individual QDs immobilized onto dielectric surfaces, such as glass coverslip. It has also been shown that local environments play a key role in this blinking phenomena and can affect the emission properties of QDs (8-13). Surrounding the QDs with oligo ligands(8) or thiol groups(9) suppresses the blinking. This suppression is presumably due to the passivation of surface trap by ligand groups. Enhanced luminescence in the presence of gold colloids(10,12) have also been reported recently. A recent investigation in our lab has revealed the enhanced fluorescence of home-synthesized QDs adsorpted directly on silver island film(11). Despite recent progress, much work needs to be done to achieve reproducible and robust surface fuctionalization by developing flexible bioconjugation techniques. Experimental results from various spatial configurations in addition to QD preparation conditions differ in terms of spectral properties of QDs. In addition, as the emission properties of the individual adsorbed QDs vary dramatically with respect to the assorted experiment conditions and rough metallic surface topology, it is difficult to evaluate their emission characteristics accurately. Here we describe a method to apply commercially available streptadvin-conjugated QD coupled to biotinylated BSA monolayer to achieve structural control between the QD and the metallic nanostructure. The surface derivatization of QDs with organic ligands enables the QDs to disperse in a variety of supported matrices. The use of BSA-monolayer technique offers more flexibility in spatial geometry control and provides a more convenient approach for biological application.
One important technique for the elucidation optical properties of quantum dots is single-molecule fluorescence spectroscopy, which eliminates averaging overall members of the ensemble and can reveal fundamental properties otherwise hidden in ensemble measurement. Time-dependent fluorescence fluctuations from a single QD reveal the presence of fluorescence intermittency. In this work, we reported the results of suppressed blinking of Quantum dots near SIFs.
Materials and methods
Single-molecule measurements were performed using a confocal microscopy system (MicroTime 200, Picoquant, Germany) with an excitation line at 470 nm. Images were recorded by raster scanning (in a bidirectional fashion) the sample over the focused spot of the incident laser with a pixel integration of 0.6 ms. The excitation power into the microscope was maintained less than 0.1 μW. Time-dependent fluorescence data were collected with a dwell time of 50 ms. The fluorescence lifetimes of single molecules were measured by time-correlated single photon counting (TCSPC) with the TimeHarp 200 PCI-board (PicoQuant). The data was stored in the time-tagged-time-resolved (TTTR) mode, which allows recording every detected photon with its individual timing information. In combination with a pulsed diode laser, Instrument Response Function (IRF) widths of about 300 ps FWHM can be obtained, which permits the recording of sub-nanosecond fluorescence lifetimes, extendable to less than 100ps with reconvolution. Lifetimes were estimated by fitting to a χ2 value of less than 1.2 and with a residuals trace that was fully symmetrical about the zero axis. All measurements were performed in a dark compartment at room temperature. Silver island films (SIFs) were deposited on cleaned glass coverslips by reduction of silver nitrate as reported previously(14). The formed silver island films are greenish and non-continuous. Only one side of each slide was coated with SIF. The particles are typically 100−500 nm across and 70 nm high covering about 20% of the surface(15). The QDs immobilization procedure is illustrated in Scheme 1. A solution of 1mg/mL biotinylated BSA (Sigma) in 0.1× PBS (pH 7.2) was applied onto the surface and the slide was incubated in a humid chamber overnight at 5 °C, then extensively washed with distilled water. In a following step, a solution of streptavidin-conjugated CdSe/ZnS quantum dot (Qdot 655, Quantum Dot Corp.) was deposited and the slide was incubated for 1 hour. The QD concentration was adjusted at nanomolar levels (0.1∼0.5 nM) to give an appropriate surface density for single-molecule studies. After incubation, the dye-immobilized coverslip was thoroughly rinsed with HEPEs buffer solution to remove loosely bound QD molecules and washed extensively with 0.1× PBS buffer.
Scheme 1.
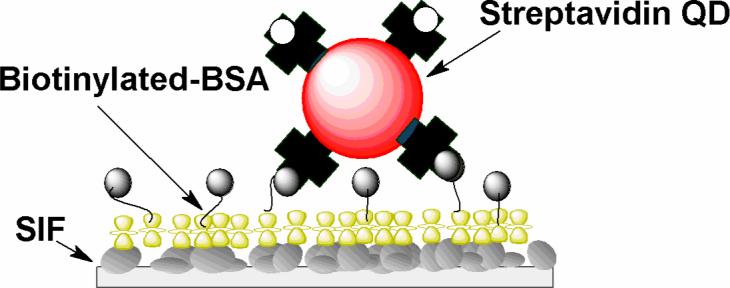
Immobilization of streptavidin conjugated quantum dots on biotinylated BSA monolayer. (the glass surface was coated with silver island film).
Results and Discussion
The blinking phenomenon is confirmed by the single molecule fluorescence images. During the raster scan, the bright spots in the image demonstrate the characteristic fluorescence intermittency associated with single quantum dots immobilized on a BSA-coated glass coveslip. As clearly illustrated in Figure 1A, a single molecule stops emitting fluorescence, the “off” time can be observed as a dark stripe in the spot of the image and followed by reoccurrence of the florescence (“on” time). On the contrary, one can clearly observe that whereas some molecules emit nearly continuously in the presence of silver island films (Figure 1).
Figure 1.
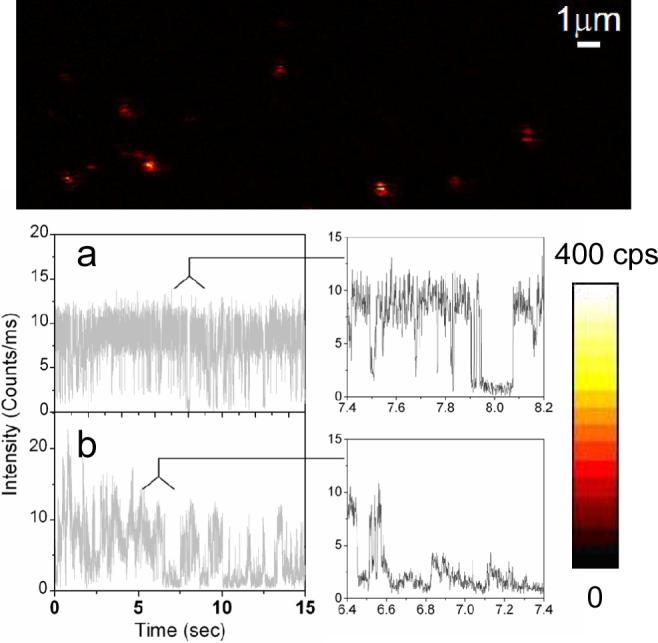
Single molecule fluorescence images (10×10 μm) and representative fluorescence time traces and their expanded sections recorded for single QDs immobilized on glass substrates. Each image pixel has a 0.6 ms dwell time and the fluorescence intensity is displayed in a colorized scale, ranging from dark to light.
Figure 1 displays typical time traces of the fluorescence intensity from single strepavidin QDs bound to a BSA-biotin layer on glass substrate. In Figure 1a, under continuous illumination, the single QD switch abruptly between a fluorescent “on” state and a non-fluorescent “off” or weak fluorescent “dim” state. Two intensity levels are clearly distinguishable beyond those expected from shot-noise limited fluctuations. Another representative emission pattern is illustrated in Figure 1b while the emission fluctuates stochastically compared to Figure 1a. In the presence of metallic nanostructure, a large distribution of quantum dots shows as much as more than 3-fold increase in fluorescence intensity. In addition, a large fraction of molecule studied (>70%) did not display any long off times of interest. The time traces observed from SIF show relatively continuous rather than discrete intensity fluctuations (Figure 2). The interval of “on” times increases to a point where the blinking events rarely appears during the illumination. As illustrated in Figure 2b, the fluorescence intensity is well above the background noise level, we do not observe dark states during the 60-second observation time. To examine blinking on the large timescale of many seconds, a threshold was set at a level of 3-fold standard deviation with respect to the mean background during the on/off histogram analysis to distinguish between the states as described previously(16,17). We find that the decay of probability density for single QDs on glass is linear on a log-log scale and can be fit by an inverse power-law kinetics, which has been intensively discussed to describe the quantum dot blinking(5-7,18,19). The approximately linear fit yields a value of 1.74 (Figure 3), which resembles previous data(6,7). Similarly, we observed a roughly fit of off-time distribution for single QDs in the presence of SIFs with a value of 2.50. A difference in the local environment and fluctuations of the QD is expected to be reflected in the blinking coefficients αoff(20).
Figure 2.
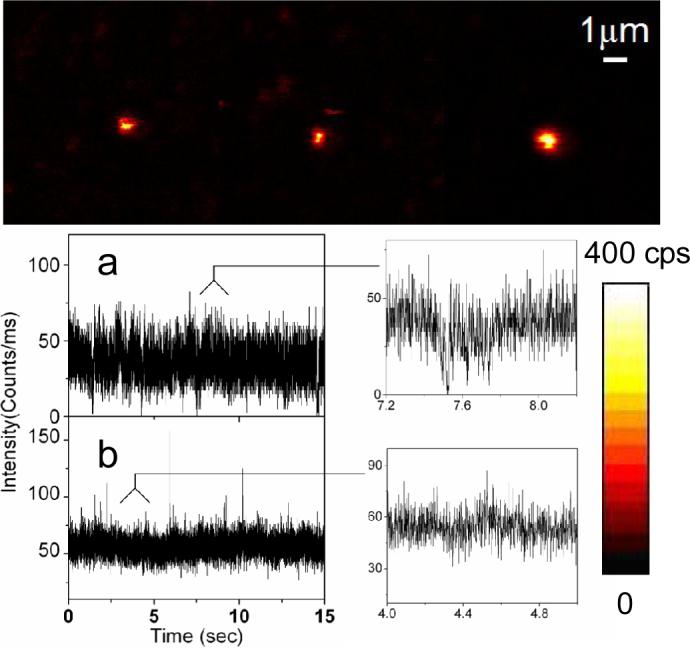
Single molecule fluorescence images (10×10 μm) and representative fluorescence time traces and their expanded sections recorded for single QDs immobilized on SIF substrates. Each image pixel has a 0.6 ms dwell time and the fluorescence intensity is displayed in a colorized scale, ranging from dark to light.
Figure 3.
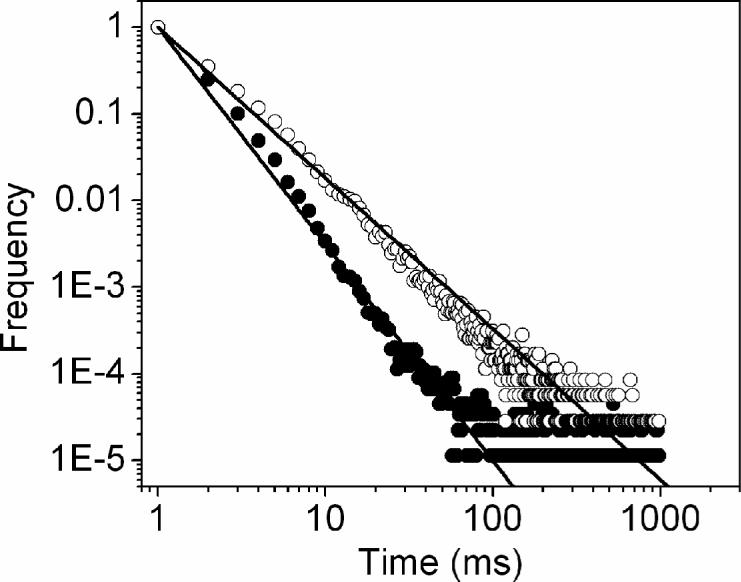
“off” time histograms compiled from around 70 single QD molecules. The lines are approximately inverse power-law fits to the histogram, giving rise to an exponent of −1.74 on glass (open circle) and −2.5 on SIF (solid circle).
There exist several possible mechanisms to explain the nature of fluorescence intermittency in semiconducting QDs. One popular explanation for the inverse power law behavior of the “off” time is the presence of multiple ionization states and accordingly a distribution of recombination rates (5-7). As an “off” state is ascribed to be caused by a photo-induced process of the QD, the “off” time intervals are associated with the recombination rate of the ejected charge carrier (most likely electrons) with the ionized dot. The “off” state happens when one of the electrons in the photo-excited exciton either becomes trapped at the QD surface with some probability to reversibly neutralize the quantum dot after some time, or tunnels into the surrounding environment. The ionized quantum dot appears to be dark. Emission resumes once the trapped charge carrier returns to a delocalized state in the core or a nearby electron is captured from the surrounding environment. This model predicts the long-lived trap state, thereby well-defined sets of on/off durations should be observed regularly in the emission time transients(18,21). However the fact that a large fraction (∼65%) of single QDs displayed stochastic fluctuations as depicted in Figure 1b, indicating that such long-live ionized state model can not explain well with the observed fluorescence intermittency. Recently, Frantsuzov and Marcus(22) presented an alternative mechanism in their discussion of the correlation between the non-radiative relaxation and the blinking of neutral QDs. In this model, the on/off blinking arises from fluctuations in the non-radiative relaxation rate when the excited QD returns directly (or via a surface state) back to its ground state. The hole trapping is an Auger process. The extra hole energy enables the electron excitation from 1Se state to 1Pe state. The energy gap between 1Pe and 1Se states is a stochastic process. It acts as a slow variable and creates large variations in the trapping rate, which can be reflected as stochastic fluorescence intensity fluctuation clearly observed in this experiment.
The large disparity of off-time distributions we observed in Figure 3 is likely caused by the changes in non-radiative decay channels due to the effect of surrounding metallic nanostructure. The metallic structure with the sub-wavelength size usually displays an energy resonance arising from the collective oscillation of migrated electrons on its surfaces, which is defined as plasmon resonance(23,24). An emitter is described as an oscillating dipole to radiate energy when emission occurs. When the dipole is localized near the metal particle, the radiating energy from the fluorophore is dramatically altered through coupling with the metal plasmon resonance to cause a change of the emission properties, which is referred as Radiative Decay Engineering (RDE)(15). These energy resonances express as absorbance, excitation, and scattering and induce strongly enhanced local fields in a near field region spatially overlap with QDs. The highly enhanced scattering fields can be adsorbed by the QDs. As a result, the plasmon interaction could amend the energy gap between the exciton hole and trapped hole, and thus eliminates hole-trapping process, leading to reduce the frequency of blinking. However, the plasmonic effect on energy separations between the states is varied, for instance, due to heterogeneous distributions of plasmon field, accounting for the remaining blinking events.
The fluorescence lifetime is sensitive to the local environment of the molecule. The decay time is often mono-exponential and given by
| (1) |
where Γ and knr are the radiative and non-radiative decay rate, respectively. The changes in knr are typically due to changes in an emitter's environment, quenching or RET. The radiative decay rate Γ is constant and any changes are primarily due to changes in refractive index. Proximity of quantum dots to metals can result in an increase in the total radiative decay rate(15). The lifetime and the quantum yield near the metal then become
| (2) |
| (3) |
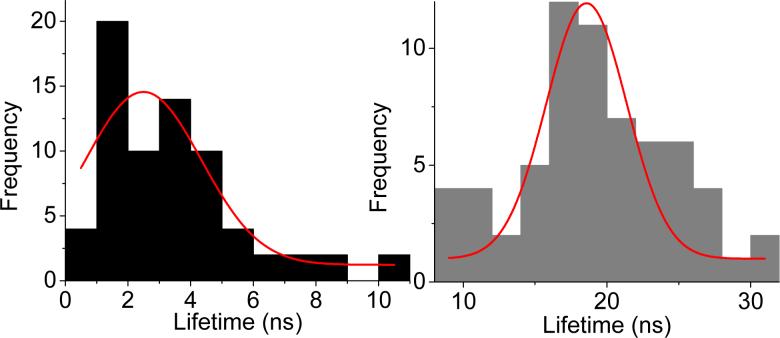
The radiative rate is given by Γ + Γm, when Γm is the part due to the metal. The change in radiative rate results in remarkable effects such as the increase in quantum yield and decrease in lifetime. We implemented the time-correlated single photon counting (TCSPC) measurement on a single bright spot. Figure 4 illustrate histograms of lifetimes acquired from single QDs on the unsilvered surface (gray) and silvered surface (black), respectively. Each distribution contains data from approximately 70 molecules and is fit to a Gaussian function. The mean of the lifetime distribution on glass is 19.5 ns. In the presence of SIFs, the lifetime distribution shows a long tail and yields a mean value of 2.4 ns. The decrease in lifetime implies that that the radiative rate of the quantum dot is much larger on the SIF than on glass(14,15,25,26), which is compatible with overall increased intensity and reduced “off” time. The wide distributions of lifetimes can be viewed as the effect of the fluctuations of the nonradiative relaxation rate postulated by Frantsuzov and Marcus(22). The unsymmetrical distribution of lifetimes on silvered surface could also arise from diverse metal-fluorophore interactions that may be due to the heterogeneous surface topography of metallic nanostructure(25,27,28).
Figure 4.
Lifetime distributions of single QDs(Black: on SIFs; gray: on glass. Red Lines: Gaussian fits)
In conclusion, we observed the suppressed blinking of single Quantum dot immobilized near silver island films. The “off” time distribution of quantum dots near SIFs displayed power law dependence with a dissimilar exponent compared with that of electromagnetic inert surface. We ascribe it to the modification of fluorescence by the surface plasmon resonance from the metallic nanostructure. The hole-trapping process is dramatically altered through coupling with the metal plasmon resonance to cause a change in the fluorescence properties and lead to suppression of blinking. The strong interaction of the excited molecules with the metal nanostructures also dramatically shortens the lifetime of the non-emissive state. The individual QDs showed the extended “on” time and overall increased intensity near metallic nanostructures. The findings should make the use of single QDs for a variety of applications in light-emitting devices.
ACKNOWLEDGMENTS
This research was supported by grants from NIH, HG-02655, EB-00682, and NCRR, RR-08119.
Appendix
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES.
- 1.Bruchez M, Moronne M, Gin P, Weiss S. AP Alivisatos: Semiconductor nanaocrystas as fluorescent biological labels. Science. 1998;281:2013–16. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 2.Chan W, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–18. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 3.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaing, labelling and sensing. Nature Materials. 2005;435−446 doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C-Y, Yeh H-C, Kuroki MT, Wang T-H. Single-quantum-dot-based DNA nanosensor. Nature Materials. 2005;4:826–31. doi: 10.1038/nmat1508. [DOI] [PubMed] [Google Scholar]
- 5.Haase M, Hubner CG, Reuther E, Herrmann A, Mullen K, Basche T. Expoential and power-law kinetics in single-molecule fluorescence intermittency. J. Phys. Chem. B. 2004;108 [Google Scholar]
- 6.Kuno M, Fromm DP, Hamann HF, Gallagher A, Nesbitt DJ. Nonexponential “blinking” kinetics of single CdSe quantum dots: a universal power law havior. Journal of Applied Physics. 2000;112:3117–20. [Google Scholar]
- 7.Kuno M, Fromm DP, Hamann HF, Gallagher A, Nesbitt DJ. “On”/“off” fluorescence intermittency of single semiconductor quantum dots. J. Chem. Phys. 2001;115:1028–40. [Google Scholar]
- 8.Hammer NI, Early KT, Sill K, Odoi MY, Emriek T, Barnes MD. Coverage-Mediated Suppression of Blinking in Solid State Quantum Dot Conjugated Organic Composite Nanostrutures. J. Phys. Chem. B. 2006;110:14167–71. doi: 10.1021/jp062065f. [DOI] [PubMed] [Google Scholar]
- 9.Hohng S, Ha T. Near-complete suppression of quantum dot blinking in ambient conditions. J. Am. Chem. Soc. 2004;126:1324–25. doi: 10.1021/ja039686w. [DOI] [PubMed] [Google Scholar]
- 10.Kulakovich O, Strekal N, Yaroshevich A, Maskevich S, Gaponenko S, Nabiev I, Woggon U, Artemyev M. Enhanced Luminescence of CdSe Quantum dots on Gold Colloids. Nano Letters. 2002;2:1449–52. [Google Scholar]
- 11.Ray K, Badugu R, Lakowicz JR. Metal-Enhanced Fluorescence from CdTe Nanocrystals: A Single-Molecule Fluorescence Study. J. Am. Chem. Soc. 2006;128:8898–999. doi: 10.1021/ja061762i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu KT, Woo WK, Fisher BR, Eisler HJ, Bawendi MG. Surface-enhanced emission from single semiconductor nanocrystals. Physical Review Letters. 2002;89:117401–1-17401-4. doi: 10.1103/PhysRevLett.89.117401. [DOI] [PubMed] [Google Scholar]
- 13.Song J-H, Atray T, Shi S, Urabe H, Nurmikko AV. Large Enhancement of Fluorescence Efficiency from CdSe/ZnS Quantum Dots Induced by Resonant Coupling to Spatially Controlled Surface Plasmons. Nano Letters. 2005;5:1557–61. doi: 10.1021/nl050813r. [DOI] [PubMed] [Google Scholar]
- 14.Lakowicz JR, Malika J, Gryczynski I, Gryczynski Z, Geddes C. Radiative Decay Engineering: the Role of Photonic Mode Density in Biotechnology. J. of Phys. D: Appl.Phys. 2003;36:R240–R49. doi: 10.1088/0022-3727/36/14/203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakowicz JR. Radiative Decay Engineering: Biophysical and Biomedical Applications. Anal. Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weston KD, Carson PJ, Metiu H, Buratto SK. Room-temperature fluroescence characteristics of single dye molecules adsorbed on a glass surface. J. Chem. Phys. 1998;109:7474–85. [Google Scholar]
- 17.Panzer O, Gohde W, Fischer UC, Fuchs H, Mullen K. Influence of oxygen on single molecule blinking. Advanced Materials. 1998;10:1469–72. [Google Scholar]
- 18.Krogmeier JR, Hwang J. Data analysis considerations in probing single quantum dot fluorescence intermittency. Proceedings of SPIE. 2005;5705:255–62. [Google Scholar]
- 19.Stefani FD, Zhong X, Knoll W, Han M, Kreiter M. Memory in quantum-dot photoluminescence blinking. New J. Phys. 2005;7:1–17. [Google Scholar]
- 20.van Sark WGJHM, Frederix PLTM, Bol AA, Gerritsen HC, Meijerink A. Blueing, bleaching, and blinking of single CdSe/ZnS quantum dots. ChemPhysChem. 2002;3 [Google Scholar]
- 21.Biebricher A, Sauer M, Tinnefeld P. Radiative and Nonradiate Rate Fluctuations of Single Colloidal Seiconductor Nanocrystals. J. Phys. Chem. B. 2006;110:5174–78. doi: 10.1021/jp060660b. [DOI] [PubMed] [Google Scholar]
- 22.Frantsuzov PA, Marcus RA. Explanation of quantum dot blinking without the long-lived trap hypothesis. Physical Review B. 2005;72:155321. [Google Scholar]
- 23.Antunes PA, Constantino CJL, Aroca RF, Duff J. Langmuir and Langmuir-Blodgett Films of Perylene Tetracarboxylic Derivatives with Varying Alkyl Chain Length: Film Packing and Surface-Enhanced Fluorescence Studies. Langmuir. 2001;17:2958–64. [Google Scholar]
- 24.Kumbhar AS, Kinnan MK, Chumanov G. Multiple Plasmon Resonances of Submicron Silver Particles. J. Am. Chem. Soc. 2005;127:12444–45. doi: 10.1021/ja053242d. [DOI] [PubMed] [Google Scholar]
- 25.Malicka J, Gryczynski I, Fang J, Kusba J, Lakowciz JR. Photostability of Cy3 and Cy5-Labeled DNA in the Presence of Metallic Silver Particles. J. of Fluorescence. 2002;12:439–47. doi: 10.1023/A:1021370111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efros AL, Rosen M, Kuno M, Nirmal M, Noris DJ, Bawendi M. Band-edge exciton in quantum dots of semiconductors with a degenerate valence band: Dark and bright exciton states. Physical Review B. 1996;54:4843–56. doi: 10.1103/physrevb.54.4843. [DOI] [PubMed] [Google Scholar]
- 27.Aussenegg FR, Leitner A, Lippotch ME, Reinisch H, Reigler M and. Enhanced dye fluorescence over silver island films: analysis of the distance dependence. Surface Sci. 1987;139:935–45. [Google Scholar]
- 28.FU Y, Lakowicz JR. Enhanced Fluorescence of Cy5-Labeled DNA Tethered to Silver Island Films: Fluorescence Images and Time-Resolved Studies Using Single-Molecule Spectroscopy. Anal. Chem. 2006;78:6238–45. doi: 10.1021/ac060586t. [DOI] [PMC free article] [PubMed] [Google Scholar]