Abstract
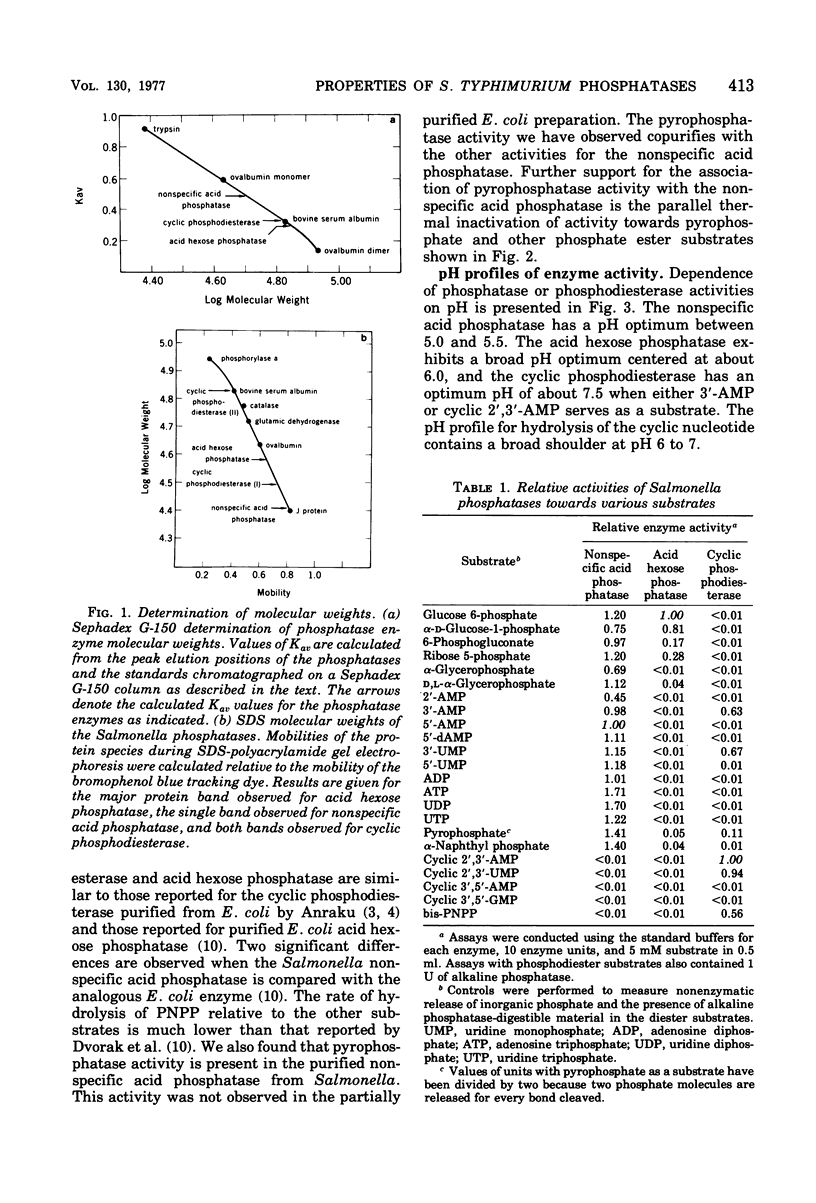
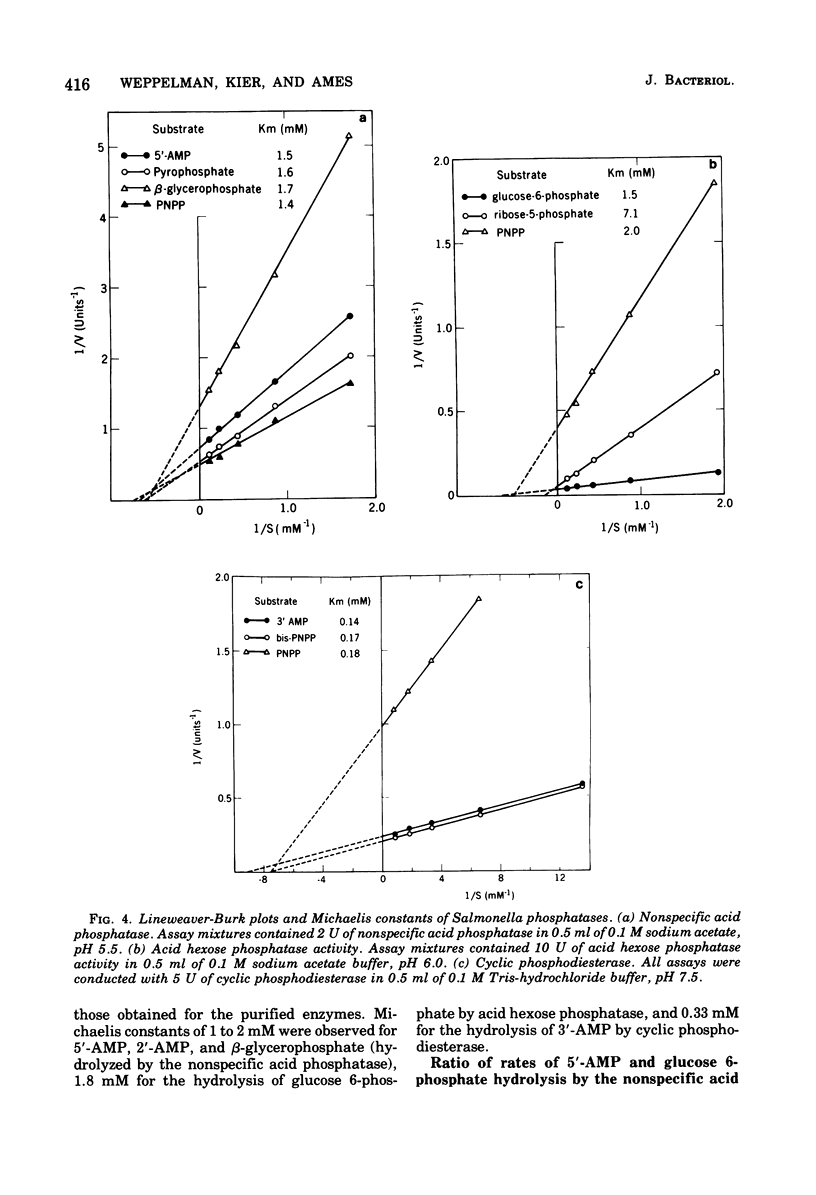
The properties of three phosphatases from Salmonella typhimurium have been examined. A cyclic 2',3'-nucleotide phosphodiesterase (EC 3.1.4.d) hydrolyzes cyclic 2',3'-purine and -pyrimidine nucleotides, as well as 3'-mononucleotides, and has a pH optimum of about 7.5. It requires divalent cations for activity and has a molecular weight of 67,000. Acid hexose phosphatase (EC 3.1.2.2) possesses activity towards hexose phosphates as well as other sugar phosphates. The enzyme is apparently a dimer of 37,000-dalton subunits. Nonspecific acid phosphatase (EC 3.1.3.2) hydrolyzes a variety of phosphate esters, including nucleotides and sugar phosphates. The enzyme also hydrolyzes the phosphoric anhydride bonds of pyrophosphate and nucleotides. Michaelis constants of the nonspecific acid phosphatase for several of its substrates are in the 1 to 2 mM range. Nonspecific acid phosphatase is a dimer of 27,000-dalton subunits.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Oct;239:3412–3419. [PubMed] [Google Scholar]
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. II. FURTHER STUDIES ON SUBSTRATE SPECIFICITY AND MODE OF ACTION OF THE ENZYME. J Biol Chem. 1964 Oct;239:3420–3424. [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Carrillo-Castañeda G., Ortega M. V. Effect of inorganic phosphate upon Salmonella typhimurium phosphatase activities: non-respressible alkaline phosphatase and non-inhibited acid phosphatase. Biochim Biophys Acta. 1967;146(2):535–543. doi: 10.1016/0005-2744(67)90237-9. [DOI] [PubMed] [Google Scholar]
- Chakraburtty K., Burma D. P. The purification and properties of a ribonuclease from Salmonella typhimurium extract. J Biol Chem. 1968 Mar 25;243(6):1133–1139. [PubMed] [Google Scholar]
- Dvorak H. F., Brockman R. W., Heppel L. A. Purification and properties of two acid phosphatase fractions isolated from osmotic shock fluid of Escherichia coli. Biochemistry. 1967 Jun;6(6):1743–1751. doi: 10.1021/bi00858a024. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Heppel L. A. Metallo-enzymes released from Escherichia coli by osmotic shock. II. Evidence that 5'-nucleotidase and cyclic phosphodiesterase are zinc metallo-enzymes. J Biol Chem. 1968 May 25;243(10):2647–2653. [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R., Ames B. N. Regulation of two phosphatases and a cyclic phosphodiesterase of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):420–428. doi: 10.1128/jb.130.1.420-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pogell B. M., Maity B. R., Frumkin S., Shapiro S. Induction of an active transport system for glucose 6-phosphate in Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):406–415. doi: 10.1016/0003-9861(66)90047-6. [DOI] [PubMed] [Google Scholar]
- Rodden J. L., Scocca J. J. Purification and properties of cyclic phosphodiesterase: 3'-nucleotidase, a periplasmic enzyme of Haemophilus influenzae. Arch Biochem Biophys. 1972 Dec;153(2):837–844. doi: 10.1016/0003-9861(72)90406-7. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., AMES B. N. PHOSPHORIBOSYLADENOSINE MONOPHOSPHATE, AN INTERMEDIATE IN HISTIDINE BIOSYNTHESIS. J Biol Chem. 1965 Jul;240:3056–3063. [PubMed] [Google Scholar]
- Schlesinger M. J., Reynolds J. A., Schlesinger S. Formation and localization of the alkaline phosphatase of Escherichia coli. Ann N Y Acad Sci. 1969 Oct 14;166(2):368–379. doi: 10.1111/j.1749-6632.1969.tb46408.x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wehr C. T. Isolation and properties of a ribonuclease-deficient mutant of Salmonella typhimurium. J Bacteriol. 1973 Apr;114(1):96–102. doi: 10.1128/jb.114.1.96-102.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



