Abstract
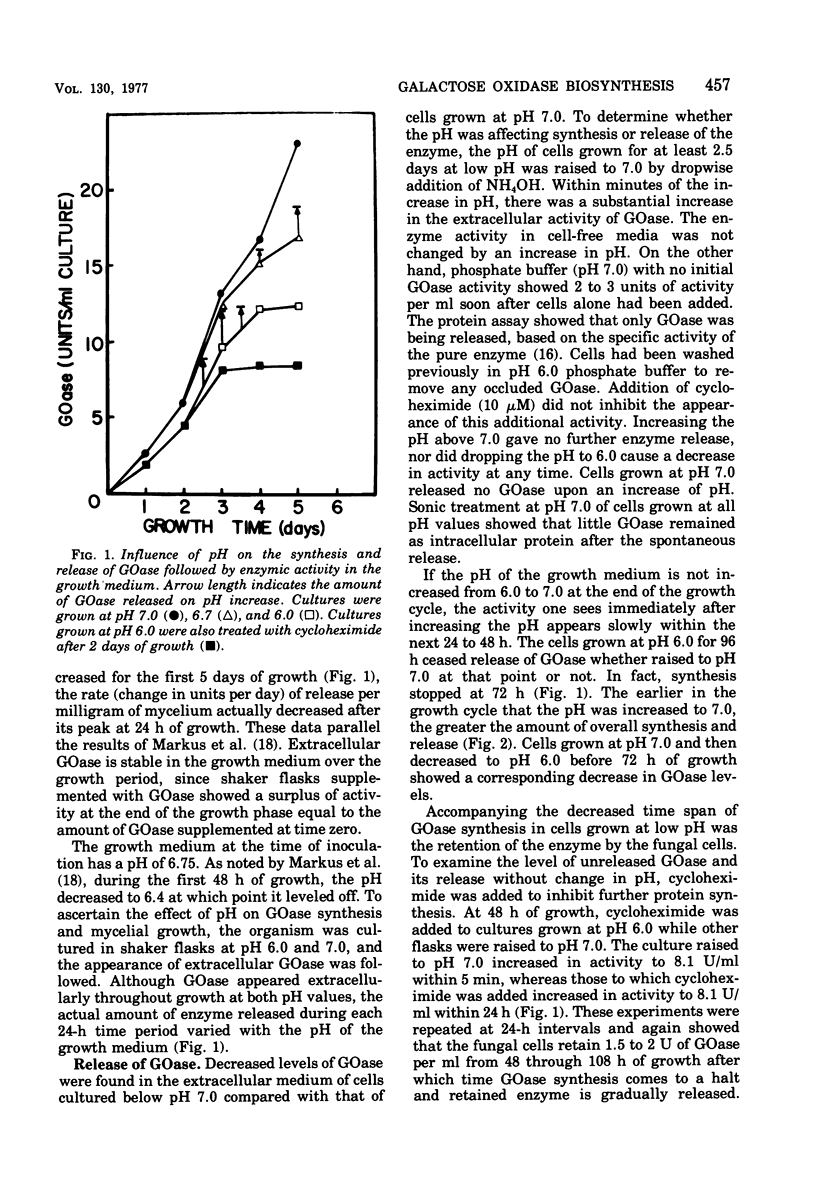
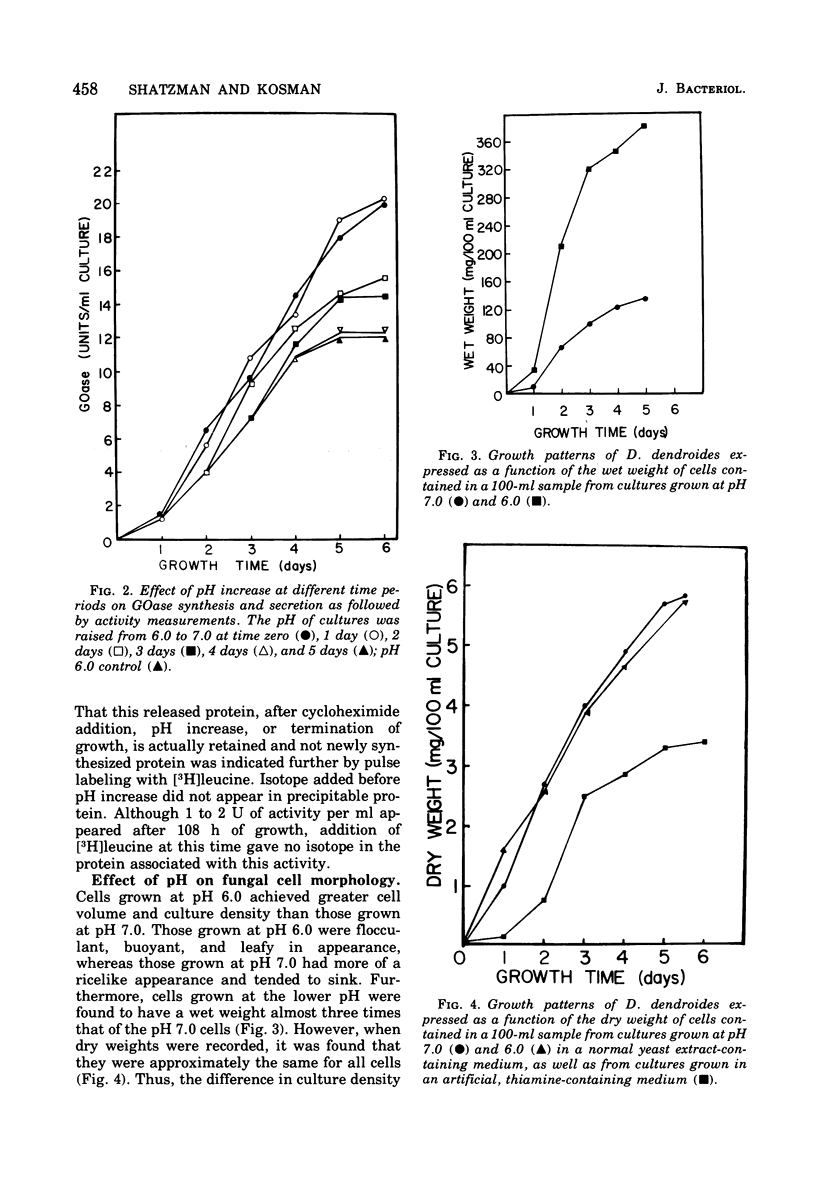
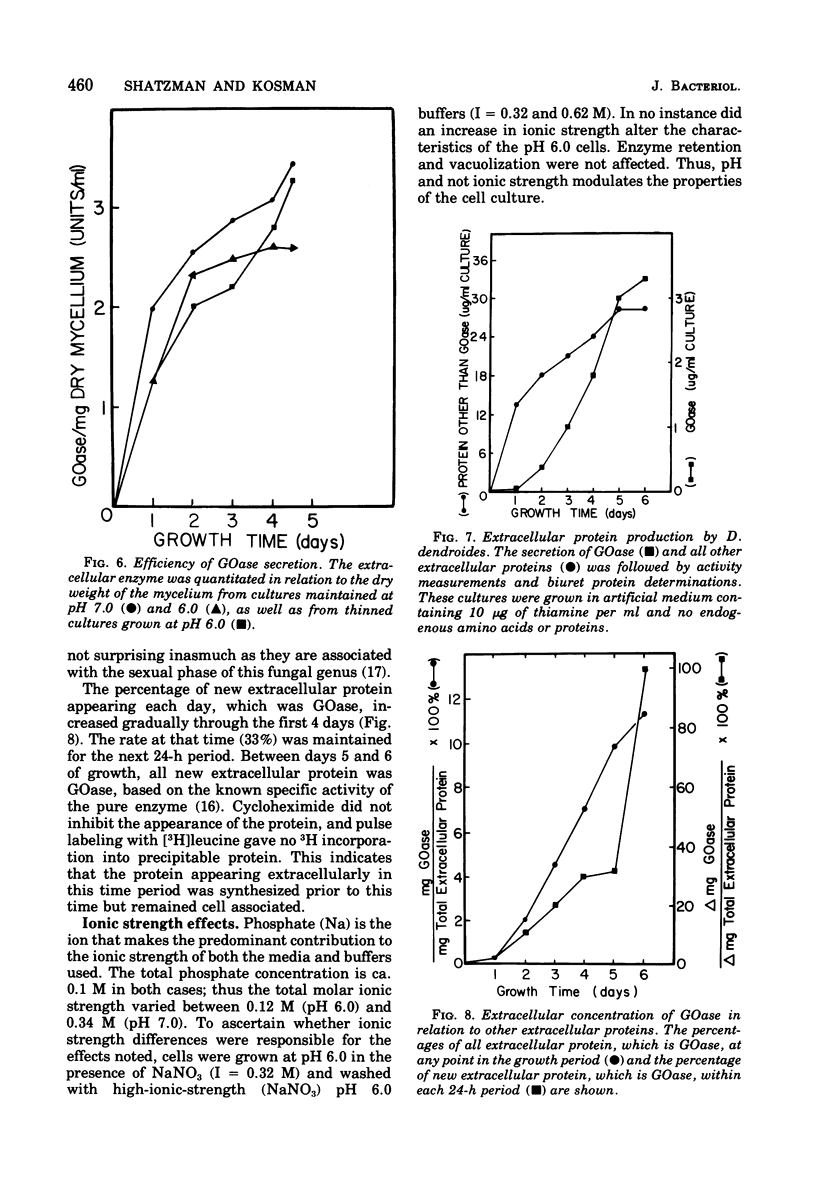
The effects of pH and growth density on the amount of an extracellular enzyme, galactose oxidase, synthesized by the fungus Dactylium dendroides were studied. Growth at a pH below 6.7 caused a decrease in the ability of the organism to release galactose oxidase. The enzyme retained by these fungal cells was liberated whenever the pH was raised to 7.0. Cycloheximide addition failed to inhibit the appearance of this protein; [3H]leucine added prior to pH adjustment was not incorporated into the released protein, These observations indicate the released protein is not newly synthesized protein. The retained enzyme would be secreted slowly over a 2-day period if the pH was not increased. In addition to regulating protein retention, pH was also shown to be associated with vacuolization, cell volume, culture density, and inhibition of protein synthesis. Cultures maintained at low pH were characterized by a dense growth consisting of highly vacuolated, buoyant, fungal hyphae. Increasing the pH from 6 to 7 caused a decrease in vacuole size. Cells grown at neutral pH maintained a lower density of growth and, based on activity measurements, synthesized 33% more galactose oxidase. Furthermore, cultures grown at pH 6.0 and maintained at a lower cell density produced galactose oxidase at a level similar to that of cells grown at neutral pH. Thus, the elevated density of the cell culture was inhibitory to galactose oxidase synthesis. The observed effects on protein synthesis and release were rather specific for galactose oxidase, since other extracellular proteins appeared in the earliest stages of growth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G., AMARAL D., ASENSIO C., HORECKER B. L. The D-galactose oxidase of Polyporus circinatus. J Biol Chem. 1962 Sep;237:2736–2743. [PubMed] [Google Scholar]
- Abd-el-Al A. T., Phaff H. J. Exo-beta-glucanases in yeast. Biochem J. 1968 Sep;109(3):347–360. doi: 10.1042/bj1090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. J., Catley B. J., Awad W. M., Jr Extracellular and protease-released pullulanases. J Bacteriol. 1976 Feb;125(2):501–508. doi: 10.1128/jb.125.2.501-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: metabolic requirements of the process. J Bacteriol. 1975 Jun;122(3):1117–1125. doi: 10.1128/jb.122.3.1117-1125.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBERHART B. M. Exogenous enzymes of Neurospora conidia and mycelia. J Cell Comp Physiol. 1961 Aug;58:11–16. doi: 10.1002/jcp.1030580103. [DOI] [PubMed] [Google Scholar]
- Finn G. J., Condon S. Regulation of catalase synthesis in Salmonella typhimurium. J Bacteriol. 1975 Aug;123(2):570–579. doi: 10.1128/jb.123.2.570-579.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C., Asensio C. Widespread occurrence of galactose oxidase and glucose oxidase in fungi. Arch Biochem Biophys. 1967 Mar;119(1):588–590. doi: 10.1016/0003-9861(67)90498-5. [DOI] [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Release of extracellular enzymes from Bacillus amyloliquefaciens. J Bacteriol. 1975 Apr;122(1):34–40. doi: 10.1128/jb.122.1.34-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Cell wall alterations associated with the hyperproduction of extracellular enzymes in Neurospora crassa. J Bacteriol. 1972 Aug;111(2):443–446. doi: 10.1128/jb.111.2.443-446.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E., Morré D. J. Cytomembrane differentiation in the endoplasmic reticulum-Golgi apparatus-vesicle complex. Science. 1968 Jul 12;161(3837):171–173. doi: 10.1126/science.161.3837.171. [DOI] [PubMed] [Google Scholar]
- Hänssler G., Maxwell D. P., Maxwell M. D. Demonstration of acid phosphatase-containing vacuoles in hyphal tip cells of Sclerotium rolfsii. J Bacteriol. 1975 Nov;124(2):997–1006. doi: 10.1128/jb.124.2.997-1006.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus Z., Miller G., Avigad G. Effect of culture conditions on the production of D-galactose oxidase by Dactylium dendroides. Appl Microbiol. 1965 Sep;13(5):686–693. doi: 10.1128/am.13.5.686-693.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan R. A., Bal A. K. Cellulase localization in hyphae of Achlya ambisexualis. J Bacteriol. 1974 Feb;117(2):840–843. doi: 10.1128/jb.117.2.840-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H. Quantitative Struktur-Funktions-Korrelation an Biomembranen. Prog Histochem Cytochem. 1973;5(3):1–37. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Sanders R. L., May B. K. Evidence for extrusion of unfolded extracellular enzyme polypeptide chains through membranes of Bacillus amyloliquefaciens. J Bacteriol. 1975 Sep;123(3):806–814. doi: 10.1128/jb.123.3.806-814.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. A mechanism for penicillinasesecretion in Bacillus licheniformis. Proc Natl Acad Sci U S A. 1970 Apr;65(4):962–969. doi: 10.1073/pnas.65.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Merrick J. M. Extracellular enzyme secretion by Pseudomonas lemoignei. J Bacteriol. 1974 Jul;119(1):152–161. doi: 10.1128/jb.119.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Molecular sieving by Neurospora cell walls during secretion of invertase isozymes. J Bacteriol. 1966 Oct;92(4):1010–1015. doi: 10.1128/jb.92.4.1010-1015.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]




