Abstract
Macrophages play a key role in both normal and pathological processes involving immune and inflammatory responses, to a large extent through their capacity to secrete a wide range of biologically active molecules. To identify some of these as yet not characterized molecules, we have used a subtraction cloning approach designed to identify genes expressed in association with macrophage activation. One of these genes, designated macrophage inhibitory cytokine 1 (MIC-1), encodes a protein that bears the structural characteristics of a transforming growth factor β (TGF-β) superfamily cytokine. Although it belongs to this superfamily, it has no strong homology to existing families, indicating that it is a divergent member that may represent the first of a new family within this grouping. Expression of MIC-1 mRNA in monocytoid cells is up-regulated by a variety of stimuli associated with activation, including interleukin 1β, tumor necrosis factor α (TNF-α), interleukin 2, and macrophage colony-stimulating factor but not interferon γ, or lipopolysaccharide (LPS). Its expression is also increased by TGF-β. Expression of MIC-1 in CHO cells results in the proteolytic cleavage of the propeptide and secretion of a cysteine-rich dimeric protein of Mr 25 kDa. Purified recombinant MIC-1 is able to inhibit lipopolysaccharide -induced macrophage TNF-α production, suggesting that MIC-1 acts in macrophages as an autocrine regulatory molecule. Its production in response to secreted proinflammatory cytokines and TGF-β may serve to limit the later phases of macrophage activation.
Macrophages are key cells in immune and inflammatory responses, participating in many normal biological processes including wound healing and resistance to tumors and infections. These cells are also important mediators of the pathology of a range of chronic inflammatory and fibrotic disorders such as rheumatoid arthritis, atherosclerosis and pulmonary fibrosis. The macrophage’s role in these processes is accomplished in large part through its capacity to secrete bioactive molecules including enzymes, lipids, and a wide range of cytokines. To a large extent, it is the secretion of a complex mixture of cytokines into the surrounding milieu that mediates the effects of the macrophage on surrounding cells such as lymphocytes, fibroblasts, and endothelial cells. Production of these cytokines is usually under stringent control with a requirement for cell activation prior to their local production.
To identify novel molecules participating in the local inflammatory response, a subtracted cDNA library enriched for genes associated with macrophage activation has been screened. The cDNA library was based on the human monocytoid cell line U937, which, under the influence of retinoic acid (RA), is known to assume monocyte-like characteristics (1). The activated phenotype of RA-differentiated U937 cells was then obtained by treatment with phorbol 12 myristate 13-acetate (PMA). This study reports on the identification and expression of a clone isolated from this subtraction library that was found to encode a novel transforming growth factor β (TGF-β) superfamily cytokine designated macrophage inhibitory cytokine-1 (MIC-1). Expression of MIC-1 in macrophages was induced by a wide range of activation stimuli, and recombinant MIC-1 protein was able to inhibit lipopolysaccharide (LPS)-induced TNF-α production, suggesting a role for MIC-1 in inhibition of macrophage activation.
METHODS
Isolation of MIC-1 cDNA.
A subtracted cDNA library enriched for macrophage activation-associated genes was prepared from U937 myelomonocytic cells using the Subtracter Kit (Invitrogen) as previously described (2). The driver cDNA library was synthesized from U937 cells differentiated with 1 μM trans retinoic acid (RA) for 3 days. The tester cDNA library was prepared from U937 differentiated in the same way and then activated with 160 nM PMA for 3 hr. The clone denoted MIC-1 was selected from this library by screening as previously described (2).
DNA Sequencing.
DNA sequencing was carried out by the dideoxy chain termination method (3) using both the T7 DNA polymerase sequencing system (Promega) or the dye terminator cycle sequencing kit (Perkin–Elmer) and the Applied Biosystems 373 automated sequencer according to manufacturers’ instructions.
Cell Culture.
The human myelomonocytic cell lines U937 and KG-1 were obtained from the American Type Culture Collection and maintained in media under the recommended conditions. Human kidney cells constitutively expressing the Epstein–Barr virus EBNA-1 gene (293-EBNA) were obtained from Invitrogen and maintained as recommended.
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats by Ficoll/Paque density gradient centrifugation. Monocytes were purified from PBMC either by adherence (4, 5) or by countercurrent centrifugal elutriation (6). Culture-derived macrophages developed from these cells were cultured as described (4, 5). The cytokines TGF-β, TNF-α, interleukin 2 (IL-2), interleukin 1 (IL-1), platelet-derived growth factor (PDGF), and macrophage colony-stimulating factor (M-CSF) were all obtained from Genzyme; granulocyte–macrophage colony-stimulating factor (GM-CSF) was from Schering-Plough; and LPS, PMA, and RA were from Sigma.
293-EBNA Cell Transfection.
293-EBNA cells were transfected with the entire coding region of the MIC-1 cDNA, which had been tagged with a FLAG epitope (DYKDDDDK) just downstream of the proposed proteolytic cleavage site at amino acid 196 (pre-pro-MIC-1/FLAG). Cells were transfected with the pre-pro-MIC-1/FLAG construct DNA in the pCEP4 expression vector (Invitrogen), which was modified to remove the EBNA-1 gene. This was undertaken with lipofectamine (GIBCO/BRL) according to the manufacturer’s instructions. Transfectants were selected with 300 μg/ml hygromycin B (Boehringer Mannheim) and maintained in 200 μg/ml hygromycin B.
Immunoprecipitation, Gel Electrophoresis, and Immunoblot Analysis.
Supernatants and cell extracts were immunoprecipitated using anti-FLAG M2 affinity gel [Kodak Scientific Imaging Systems (Kodak SIS)]. Samples were analyzed by reducing or nonreducing SDS/PAGE (7) and subjected to immunoblotting with anti-FLAG M2 monoclonal antibody (Kodak SIS). Blots were developed using biotinylated anti-mouse IgG, streptavidin-peroxidase (Amersham), and Western Blot chemiluminescence reagent (DuPont/NEN).
Northern Blot Analysis.
Northern blot analysis was performed as previously described (2). Uniformity of loading and transfer was visualized by probing the blot with a 28S rRNA oligonucleotide probe: 5′-TCCGTCCGTCGTCCTCCTC-3′. Double-stranded DNA probes were random-prime-labeled with [α-32P]dCTP (Amersham), and oligonucleotides were end-labeled with [γ-32P]ATP (Amersham). Exposure was for 5 hr to overnight at −70°C using intensifying screens.
Purification of Recombinant MIC-1.
293-EBNA cells that were transfected with the pre-pro-MIC-1/FLAG construct were grown to confluence. Conditioned medium was collected over a 2-day period, and recombinant MIC-1/FLAG was purified using anti-FLAG M2 affinity gel essentially according to the manufacturer’s recommendations. Briefly, conditioned medium was incubated overnight at 4°C on a rocker platform with anti-FLAG M2 affinity gel. The gel was then washed three times with PBS, and MIC-1/FLAG was competitively eluted using FLAG peptide (Kodak SIS). The elution buffer contained 0.1% CHAPS ([3-(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate) (Sigma) to help stabilize the protein. The eluted protein was concentrated and passed through a PD-10 column (Pharmacia) to remove free peptide. Purified MIC-1/FLAG was quantitated on Western blots by comparison with FLAG-tagged bacterial alkaline phosphatase (Kodak SIS). The concentrated MIC-1/FLAG contained less than 0.01 endotoxin units/ml when assayed using the limulus lysate assay (Sigma).
Effect of MIC-1 on LPS-Induced TNF-α Production.
Monocytes purified by elutriation were aliquoted into 24-well tissue culture plates (500,000 per well). Any remaining nonadherent cells were washed off 2 hr after plating. After 18 hr the culture medium was replaced with medium containing various concentrations of MIC-1, TGF-β, or medium containing only MIC-1 elution buffer. LPS was added to cells at a final concentration of 1 μg/ml either simultaneously with the cytokines or following preincubation with the cytokines (30 min to 24 hr). The medium was collected 18 hr later for estimation of TNF-α using an ELISA kit (Genzyme) according to the manufacturer’s instructions. All experiments were repeated at least three times, and all treatments were performed in triplicate.
RESULTS
Sequence of MIC-1.
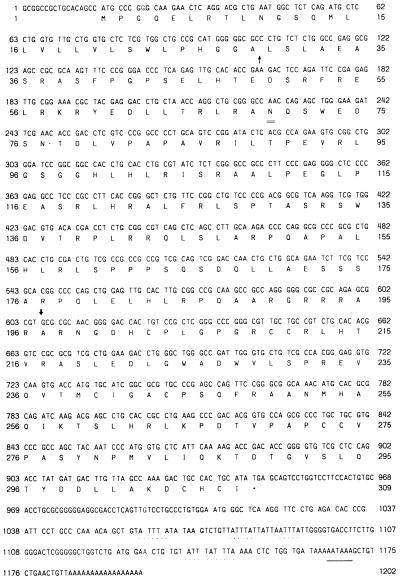
To identify macrophage activation-associated genes, a U937 cDNA subtraction library was screened. The library was constructed from RA-differentiated U937 cells and RA-differentiated PMA activated U937 cells. A clone, designated MIC-1, was isolated and further characterized by nucleotide sequencing. The 1202-bp sequence, which correlates with the size of the MIC-1 mRNA transcript (see below), contains a single, long ORF potentially encoding a protein of 308 aa (Fig. 1). The coding region is followed by a polyadenylation signal at base pair 1165, which is 20 nt upstream of the poly(A) tail. The 3′ untranslated region contains four copies of the instability motif (ATTTA) that is present in many cytokine genes and associated with posttranscriptional regulation of the mRNA. The predicted amino acid sequence contains a hydrophobic amino-terminal region typical of a signal sequence and one potential N-linked glycosylation site at amino acid 70 (Fig. 1).
Figure 1.
Nucleotide and amino acid sequence of pre-pro MIC-1. Nucleotides and translated amino acids are numbered on both sides of the figure. The putative signal sequence cleavage site (↑), putative proteolytic cleavage site (↓), consensus N-linked glycosylation site (=), and stop codon (∗) are indicated. The polyadenylation signal (_) and mRNA instability sequences (..) are underlined.
MIC-1 Is a Novel TGF-β Superfamily Protein.
The predicted MIC-1 amino acid sequence displays significant similarity to most members of the TGF-β superfamily in the protein databases. Members of this superfamily share a number of important structural characteristics. They are dimeric secreted proteins with a long propeptide separated from the mature protein by a furin-like protease acting on a conserved RXXR sequence (8–10). There are several cleavage motifs present; however, eukaryotic expression (discussed below) suggests that cleavage occurs at either 190–193 or 193–196. Cleavage usually occurs at the most downstream available site and in most superfamily members is directly followed by an alanine or serine residue (8), suggesting that cleavage occurs at amino acids 193–196 (Fig. 1), which would result in the secretion of a 224-aa dimeric mature protein.
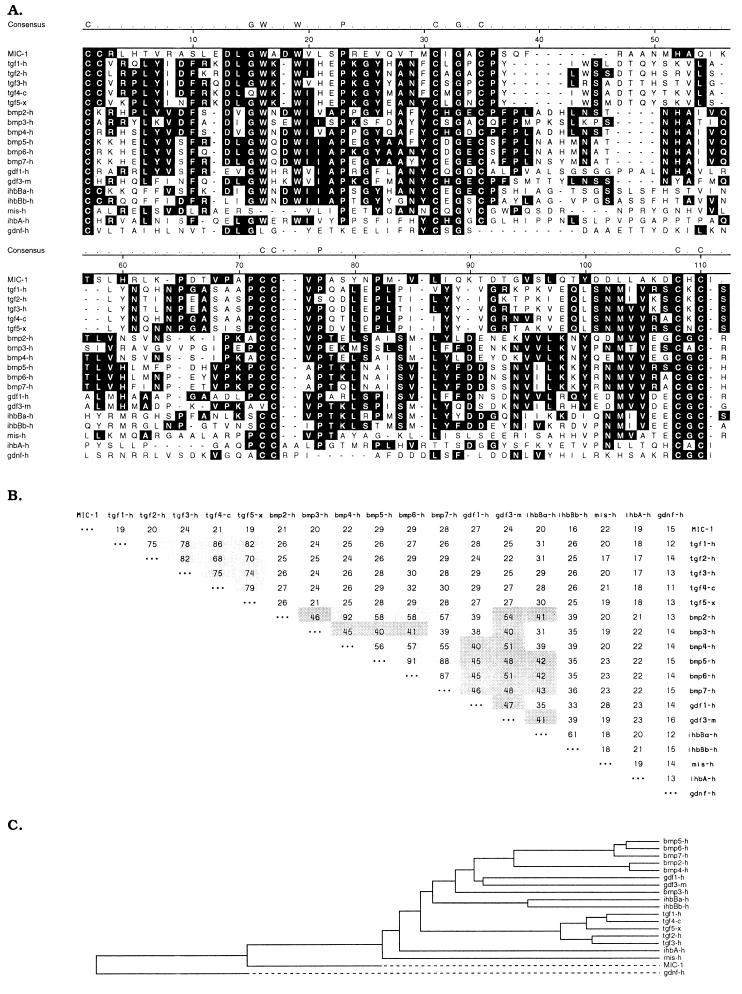
Processed TGF-β superfamily proteins all contain a highly conserved seven-cysteine domain spanning about 80 aa that encompasses most of the mature protein and forms the cysteine knot, a structural hallmark of this superfamily (8–11). To determine whether MIC-1 conformed to the structural characteristics of TGF-β superfamily proteins, multiple-sequence alignment was carried out comparing the seven-cysteine domain of MIC-1 with other proteins within the TGF-β superfamily (Fig. 2A). There is complete conservation of the cysteine residues and their spacing in all proteins including MIC-1. In addition, two other sequences conserved in many TGF-β superfamily proteins, DLGW–W and PCCVP, are both present within MIC-1 at the expected positions (Fig. 2A). This indicates that MIC-1 is a TGF-β superfamily protein.
Figure 2.
Comparison of MIC-1 amino acid sequence with members of the TGF-β superfamily. (A) Alignment of the seven-cysteine domain of MIC-1 with corresponding regions of human TGF-β1 (28), human TGF-β2 (29), human TGF-β3 (30, 31), chicken TGF-β4 (32), Xenopus TGF-β5 (33), human BMP-2,3,4 (34), human MBP-5,6,7 (35), human growth and differentiation factor (GDF)-1 (36), murine GDF-3 (11), human inhibinβ-a, βb, and α (37), human mullerian-inhibiting substance (38), and human glial cell line-derived neurotopic factor (39). Multiple-sequence alignment was performed with the dnastar program (gap weight = 10, length weight = 10). The most conserved residues, including the seven highly conserved cysteines, are identified. Dashes denote gaps introduced to optimize alignment. (B) Percent amino acid identity between the seven-cysteine domains of members of the TGF-β superfamily calculated using the pairwise sequence comparison. Darkly shaded areas denote identity of 40–54%, and lightly shaded areas, identity of >55%. (C) Dendrogram of the relationship between TGF-β superfamily members. This has been produced using the dnastar program and is based on the percentage identity.
Similar to TGF-βs 1–5 and inhibin βa and βb, MIC-1 has two extra cysteine residues close to the amino terminus of the mature peptide. In TGF-β2, these have been shown to be disulfide bonded to each other and serve to add extra stability to the finger 1 region of the molecule (12). TGF-βs 1–5 also have additional cysteine residues in the propeptide. These dimerize and associate noncovalently with the mature protein to form the latency-associated peptide, which inhibits the bioactivity of the molecule (13, 14). MIC-1 has no cysteine residues in the propeptide, indicating that there are no similar disulfide interactions in this region.
MIC-1 Is a Divergent Member of the TGF-β Superfamily.
Sequence alignments using the seven-cysteine domain are used to classify proteins within the superfamily into individual families (9–12); the major ones are TGF-β, bone morphogenetic protein (BMP), growth and differentiation factor (GDF), inhibin-β/activin (ihbB), mullerian-inhibiting substance, inhibin-α (ihbA), and glial cell line-derived neurotrophic factor. The BMP and GDF groups are sometimes clustered into a looser association known as the DVR (dpp and Vg1 related) group. A quantitative comparison of the seven-cysteine domain in MIC-1 with representative members of the TGF-β superfamily was carried out by pairwise sequence comparison using the dnastar program. The relationship between individual family members is displayed in both tabular (Fig. 2B) and dendrogram (Fig. 2C) format. As seen in Fig. 2B and as previously suggested (9, 10), members of the same family cluster together with identities of 40–90%. Members of different families bear 15–30% identity to each other. The seven-cysteine domain of MIC-1 shows 15–29% identity to the other TGF-β superfamily members (Fig. 2B), indicating that MIC-1 is a divergent member of this group that may represent the first member of a new family.
Further evidence for this was obtained by analysis of the similarity between MIC-1 and the BMPs, to which MIC-1 shows the strongest homology. The amino-terminal regions upstream of the seven-cysteine domain of the mature BMPs are all characterized by a high content of basic amino acids and are longer than the 10–14 aa seen in most other TGF-β superfamily members (8). This basic region is thought to be involved in the deposition of osteogenic proteins in bone tissue. Mature MIC-1 has only a short stretch of 14 aa preceding the seven-cysteine domain. Although this region, like other TGF-β family members, contains some basic residues, it lacks the longer stretches of highly basic regions seen in the BMPs (8).
MIC-1 DNA Encodes a Secreted Protein.
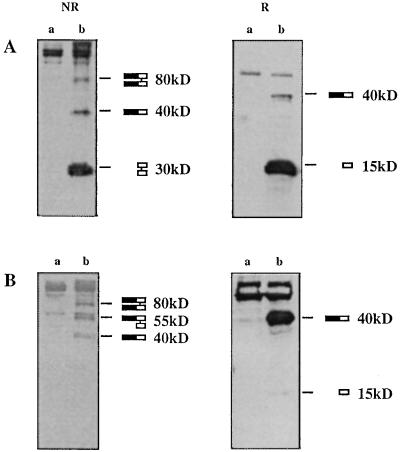
To demonstrate that MIC-1 cDNA encodes a secreted protein, 293-EBNA cells were transfected with MIC-1 cDNA that has been tagged with a FLAG epitope just downstream of the predicted proteolytic cleavage site at amino acid 196 (pre-pro-MIC-1/FLAG). Immunoprecipitation of supernatants from transfected cells with anti-FLAG antibodies followed by immunoblot analysis with anti-FLAG antibodies demonstrates that MIC-1/FLAG is secreted mainly as a 30-kDa disulfide-linked dimer (Fig. 3). Under reducing conditions the major form of MIC-1/FLAG migrates at approximately 15 kDa, correlating with the size of the mature peptide (12 kDa) predicted by cleavage at the putative proteolytic site. The slight apparent increase in size of the observed band is probably due to the presence of the FLAG epitope, which has been shown to cause excess retardation of electrophoretic mobility (15). Under nonreducing conditions, the major secreted product migrates as a 30-kDa disulfide linked dimer. Minor bands at 40 and 80 kDa may correspond to pro-MIC-1/FLAG monomer and pro-MIC-1/FLAG dimer, respectively. Analysis of cell lysates under nonreducing conditions demonstrates the presence of three forms of pro-MIC-1/FLAG precursor: 40 kDa (pro-MIC-1/FLAG monomer), 55 kDa (pro-MIC-1/FLAG hemi-dimer), and 80 kDa (pro-MIC-1/FLAG dimer). Under reducing conditions the major band migrates as the 40-kDa pro-MIC-1/FLAG monomer with a minor amount of MIC-1/FLAG monomer arising from the hemi-dimer. No mature MIC-1/FLAG peptide was observed intracellularly, suggesting rapid and efficient secretion of the mature MIC-1 peptide.
Figure 3.
Expression of pre-pro-MIC-1 cDNA in 293-EBNA cells. Supernatant (A) and total cell lysate (B) from untransfected (a) and MIC-1 transfected (b) 293-EBNA cells were immunoprecipitated with anti-FLAG antibodies and analyzed by immunoblotting with anti-FLAG antibodies under both reducing (R) and nonreducing (NR) conditions. The various forms of MIC-1 corresponding to the indicated bands are depicted on the right, with the closed bar indicating the pro-domain and the open bar indicating the mature domain.
Macrophage Activation Enhances Expression of MIC-1.
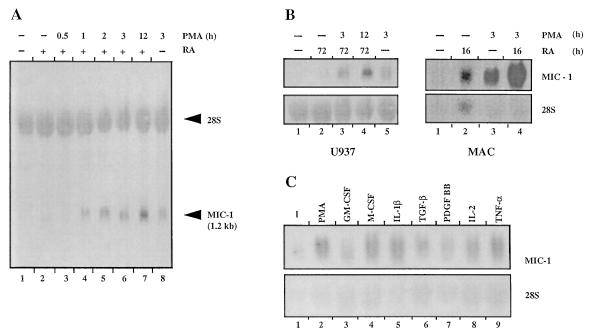
To confirm the relevance of MIC-1 to monocytoid cell activation, the expression of MIC-1 has been examined in the human monocytoid cell lines U937 and KG-1 as well as in macrophages. U937 cells were differentiated with 1 μM RA for 3 days and then activated with 160 nM PMA for 30 min to 12 hr (Fig. 4A). The MIC-1 transcript (1.2 kb) is not expressed in untreated cells, is slightly up-regulated by RA differentiation alone and up-regulated to a greater extent by treatment with PMA alone (lanes 1, 2, and 8, respectively). However, MIC-1 expression is greatly enhanced by RA differentiation followed by activation with PMA, with the level of expression increasing with duration of PMA treatment (lanes 3–7). This suggests that optimal PMA activation of MIC-1 expression requires prior differentiation of the cells by RA.
Figure 4.
Expression of MIC-1 is induced by monocyte/macrophage differentiation and activation. Northern blot analysis was performed using total RNA (20 μg per lane) and hybridization with radiolabeled MIC-1 cDNA and 28S rRNA oligonucleotide probes. (A) MIC-1 expression in U937 cells after differentiation with RA and then activation with PMA. Lanes: 1, no treatment; 2, 1 μM RA for 3 days; 3–7, 1 μM RA for 3 days followed by 160 nM PMA for 30 min, 1 hr, 2 hr, 3 hr, and 12 hr, respectively; 8, 160 nM PMA for 3 hr. (B) MIC-1 expression in U937 and macrophages following treatment with 1 μM RA for either 3 days (U937) or 16 hr (macrophages) followed by activation with 160 nM PMA (U937) or 50 nM PMA (macrophages). (C) Cytokine regulation of MIC-1 expression in macrophages. Lanes: 1, no treatment; 2, 50 nM PMA; 3, 50 units/ml GM-CSF; 4, 100 units/ml M-CSF; 5, 100 units/ml IL-1β; 6, 10 ng/ml TGF-β; 7, 10 units/ml PDGF-BB; 8, 50 units/ml IL-2; 9, 100 units/ml TNF-α. All treatments were for 3 hr.
That the level of differentiation of the cells plays a role in activation of MIC-1 expression by PMA is further supported by findings on MIC-1 expression in macrophages (Fig. 4B). MIC-1 expression in U937 cells is only slightly apparent following either RA or PMA treatment, whereas the more mature macrophages constitutively express MIC-1 mRNA at low levels. Expression in macrophages is enhanced after treatment with RA and is even more evident after PMA treatment. Expression in both U937 and macrophages is markedly enhanced after both RA and PMA treatment. In the more immature promyelocytic cell line KG-1 (16), only barely perceptible expression of MIC-1 is evident even after sequential RA/PMA treatment. Thus, induction of MIC-1 expression by RA/PMA is dependent on the level of cell differentiation, with increased responsiveness corresponding to the degree of differentiation and the capacity to express MIC-1 being a property largely of monocytes rather than more immature precursors.
To demonstrate that the activation-associated enhancement in expression of MIC-1 is not only in response to chemical activators such as PMA, macrophages were treated with a variety of cytokines involved in macrophage activation (Fig. 4C). Enhanced expression of MIC-1 was observed in particular with IL-1β, TNF-α, M-CSF, and IL-2. The effects of TGF-β, PDGF-BB, and GM-CSF are present but not as strong. Neither IFN-γ nor LPS (up to 10 μg/ml) has an effect on MIC-1 mRNA expression in macrophages (data not shown).
MIC-1 Is an Inhibitor of Macrophage Activation.
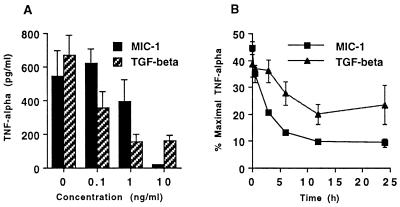
MIC-1 expression is enhanced by cytokines such as TNF-α, which is secreted by activated macrophages. This suggests that MIC-1 may function as an autocrine/paracrine regulator of macrophage activation. To determine whether MIC-1 secretion serves to limit macrophage activation, the effect of purified MIC-1 on macrophage production of TNF-α was assessed. Macrophages were incubated overnight with 0.1–10 ng/ml of MIC-1 or TGF-β1 and then activated with LPS (1 μg/ml). Conditioned medium that was harvested 18 hr later was assayed for TNF-α production by ELISA (Fig. 5A). This shows that LPS-stimulated TNF-α production was markedly suppressed in a dose–response manner by both MIC-1 and TGF-β1. Neither TGF-β1 nor MIC-1 stimulated TNF-α production when added to macrophages in the absence of LPS, and all media and diluents used exhibited no effect (data not shown). Maximal suppression requires TGF-β and MIC-1 to be added 12–24 hr prior to stimulation of macrophages with LPS (Fig. 5B). However, both cytokines still suppressed TNF-α to 40–50% of maximum when added simultaneously with LPS to macrophages (Fig. 5B).
Figure 5.
MIC-1 inhibits TNF-α production by macrophages. Macrophages were stimulated with LPS, and the effects on secretion of TNF-α were quantitated as the mean and standard deviation of triplicate cultures. Representative experiments showing the effect of MIC-1 or TGF-β1 (A) and of the preincubation period with MIC-1 or TGF-β1 (10 ng/ml) (B) on suppression of TNF-α secretion.
DISCUSSION
The TGF-β superfamily comprises an expanding group of growth and differentiation factors that has gained increasing importance over the last decade with the elucidation of their role in critical biological processes such as growth and differentiation, wound healing, and tissue repair (17, 18). The TGF-β proteins have a variety of complex pro- and antiinflammatory effects (18, 19). They play a major role in the pathogenesis of a range of important inflammatory and fibrotic disorders, including rheumatoid arthritis, atherosclerosis, pulmonary fibrosis, and cirrhosis of the liver. TGF-β1 knockout mice, however, die of severe widespread inflammation (20), which suggests that one of their major functions is antiinflammatory. On the therapeutic front, recombinant proteins, particularly from the TGF-β and BMP families, are being assessed as therapeutic agents mainly in wound and fracture healing.
Although on a structural basis a strong case can be argued that MIC-1 is a divergent member of the TGF-β superfamily, the major function of MIC-1 is not certain. However, some important clues have been obtained from studies of MIC-1 gene expression and functional studies using purified recombinant protein. The relationship of macrophage activation to MIC-1 gene expression suggests that MIC-1 may be an autocrine regulator of macrophage activation. First, the maturational state of the monocytoid cells appears important in expressing MIC-1 mRNA, as immature monocytoid cells require prior differentiation to enable MIC-1 expression. This suggests that monocyte precursors may not be able to synthesize MIC-1 in the bone marrow environment. In macrophages, MIC-1 mRNA expression is strongly induced by a number of stimuli, including PMA and a range of proinflammatory monokines. By comparison, TGF-β mRNA and biologically inactive protein are both constitutively expressed by macrophages. Induction of bioactive TGF-β, which is capable of limiting macrophage activation, occurs rapidly on exposure to activators such as LPS and IFN-γ (21, 22). Thus, TGF-β exerts its effect early during the activation process. By contrast, only minimal MIC-1 gene expression occurs in resting cells, and expression is not directly induced by LPS or IFN-γ. It is, however, induced by cytokines such as IL-1 and TNF-α, which are produced by activated macrophages, suggesting that MIC-1 may function to limit the activation process. Additionally, TGF-β itself is able to induce expression of MIC-1, suggesting the possibility that some TGF-β effects may be mediated by MIC-1.
To assess whether MIC-1, as hypothesized, could limit macrophage activation, its effect on TNF-α production by macrophages was examined. TNF-α is widely considered an important macrophage proinflammatory cytokine that causes a range of local and systemic biological effects (23, 24). TNF-α was also one of the cytokines capable of inducing MIC-1 mRNA expression in macrophages. In macrophages, LPS is one of the most potent inducers of the synthesis and secretion of this cytokine, and TGF-β has been reported to inhibit this response (25–27). Our studies indicate that MIC-1, like TGF-β, is able to markedly inhibit LPS-stimulated macrophage TNF-α secretion. Both MIC-1 and TGF-β are maximally effective if the cells are exposed to the cytokines before activation, but considerable effects are still evident if they are added simultaneously with LPS. Similarities in the dose response and periodicity would suggest that this inhibition is occurring by similar mechanisms in both TGF-β and MIC-1.
The evidence in this paper demonstrates that MIC-1 is a novel TGF-β superfamily cytokine that is able to inhibit TNF-α secretion by activated macrophages. The induction of MIC-1 mRNA by cytokines such as TNF-α and failure of induction by LPS or IFN-γ suggests that MIC-1 may not be designed to inhibit the primary activation of macrophages. Rather, it may represent an autocrine regulatory molecule whose expression may serve to limit later phases of macrophage activation by responding largely to secreted proinflammatory monokines.
Acknowledgments
This work has been funded in part by grants from St. Vincent’s Hospital and by Meriton Apartments Pty. Ltd. through a research and development syndicate arranged by Macquarie Bank Limited. We thank Drs. William Sewell and Yinon Ben-Neriah for critical reading of the manuscript.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MIC-1, macrophage inhibitory cytokine 1; TGF-β, transforming growth factor β; RA, retinoic acid; PMA, phorbol 12 myristate 13-acetate; M-CSF, macrophage colony-stimulating factor; BMP, bone morphogenetic protein; LPS, lipopolysaccharide.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF019770).
References
- 1.Harris P, Uman R P. J Leukocyte Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela S M, Martin D K, Por S B, Robbins J M, Bootcov M, Schofield P R, Campbell T J, Breit S N. J Biol Chem. 1997;272:12575–12582. doi: 10.1074/jbc.272.19.12575. [DOI] [PubMed] [Google Scholar]
- 3.Sanger F, Nicklen S, Coulson A. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett S, Por S B, Cooley M A, Breit S N. J Immunol. 1993;150:2364–2371. [PubMed] [Google Scholar]
- 5.Bennett S, Por S B, Stanley E R, Breit S N. J Immunol Methods. 1992;153:201–212. doi: 10.1016/0022-1759(92)90323-l. [DOI] [PubMed] [Google Scholar]
- 6.Wahl L M, Katona I M, Wilder R L, Winter C C, Haraoui B, Scher I, Wahl S M. Cell Immunol. 1984;85:373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- 7.Bauskin A R, Alkalay I, Ben-Neriah Y. Cell. 1991;66:685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- 8.Ozkaynak E, Schnegelsberg P, Jin D, Clifford G, Warren F, Drier E, Opperman H. J Biol Chem. 1992;267:25220–25227. [PubMed] [Google Scholar]
- 9.Burt D W, Law A S. Prog Growth Factor Res. 1994;5:99–118. doi: 10.1016/0955-2235(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 10.Kingsley D M. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 11.McPherron A, Lee S. J Biol Chem. 1993;268:3444–3449. [PubMed] [Google Scholar]
- 12.Daopin S, Piez K A, Ogawa Y, Davies D R. Science. 1992;257:369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- 13.Miyazono K, Ichijo H, Heldin C H. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- 14.Harpel J G, Metz C N, Kojima S, Rifkin D B. Prog Growth Factor Res. 1992;4:321–335. doi: 10.1016/0955-2235(92)90014-9. [DOI] [PubMed] [Google Scholar]
- 15.Knappik A, Pluckthun A. BioTechniques. 1994;17:754–761. [PubMed] [Google Scholar]
- 16.Ferrero D, Pessano S, Pagliardi G, Rovera G. Blood. 1983;61:171–179. [PubMed] [Google Scholar]
- 17.Border W A, Noble N A. New Eng J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 18.Roberts A B, Sporn M B. Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 19.Wahl S M. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Nature (London) 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assoian R K, Fleurdelys B E, Stevenson H C, Miller P J, Madtes D K, Raines E W, Ross R, Sporn M B. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunes I, Shapiro R L, Rifkin D B. J Immunol. 1995;155:1450–1459. [PubMed] [Google Scholar]
- 23.Tracey K J, Cerami A. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 24.Maini R N, Elliott M J, Brennan F M, Williams R O, Chu C Q, Paleolog E, Charles P J, Taylor P C, Feldmann M. Immunol Rev. 1995;144:195–223. doi: 10.1111/j.1600-065x.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 25.Zissel G, Schlaak J, Schlaak M, Muller-Quernheim J. Eur Cytokine Netw. 1996;7:59–66. [PubMed] [Google Scholar]
- 26.Bogdan C, Paik J, Vodovotz Y, Nathan C. J Biol Chem. 1992;267:23301–23308. [PubMed] [Google Scholar]
- 27.Chantry D, Turner M, Abney E, Feldman M. J Immunol. 1989;142:4295–4300. [PubMed] [Google Scholar]
- 28.Derynck R, Jarret J A, Chen E Y, Eaton D H, Bell J R, Assoian R K, Roberts A B, Sporn M B, Goeddel D V. Nature (London) 1985;316:701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- 29.de Martin R, Haendler B, Hofer-Warbinek R, Gavgitsch H, Wrann M, Schlusener H, Seifert J M, Bodmer S, Fontana A, Hofer E. EMBO J. 1987;6:3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ten Dijke P P, Hansen P, Iwata K K, Pieler C, Foulkes J G. Proc Natl Acad Sci USA. 1988;85:4715–4719. doi: 10.1073/pnas.85.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derynck R, Lindquist P B, Lee A, Wen D, Tam J, Graycar J L, Rhee L, Mason A J, Miller D A, Coffey R J, Moses H L, Chen E Y. EMBO J. 1988;7:3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakowlew S B, Dillard P J, Sporn M B, Roberts A B. Mol Endocrinol. 1988;2:1186–1195. doi: 10.1210/mend-2-12-1186. [DOI] [PubMed] [Google Scholar]
- 33.Kondaiah P, Sands M J, Smith J M, Fields A, Roberts A B, Sporn M B, Melton D A. J Biol Chem. 1990;265:1089–1093. [PubMed] [Google Scholar]
- 34.Wozney J M, Rosen V, Celeste A J, Mitsock L M, Whitters M J, Kriz R W, Hewick R M, Wang E A. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 35.Celeste A J, Iannazzi J A, Taylor R C, Hewick R M, Rosen V, Wang E A, Wozney J M. Proc Natl Acad Sci USA. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S J. Proc Natl Acad Sci USA. 1991;88:4250–4254. doi: 10.1073/pnas.88.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason A J, Niall H D, Seeburg P H. Biochem Biophys Res Commun. 1986;135:957–964. doi: 10.1016/0006-291x(86)91021-1. [DOI] [PubMed] [Google Scholar]
- 38.Cate R L, Mattaliano R J, Hession C, Tizard R, Farber N M, Cheung A, Ninfa E G, Frey A Z, Gash D J, Chow E P, Fisher R A, Bertonis J M, Torres G, Wallner B P, Ramachandran K L, Ragin R C, Manganaro T F, Maclaughin D T, Donahoe P K. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 39.Lin L F, Doherty D H, Lile J D, Bektesh S, Collins F. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]