Abstract
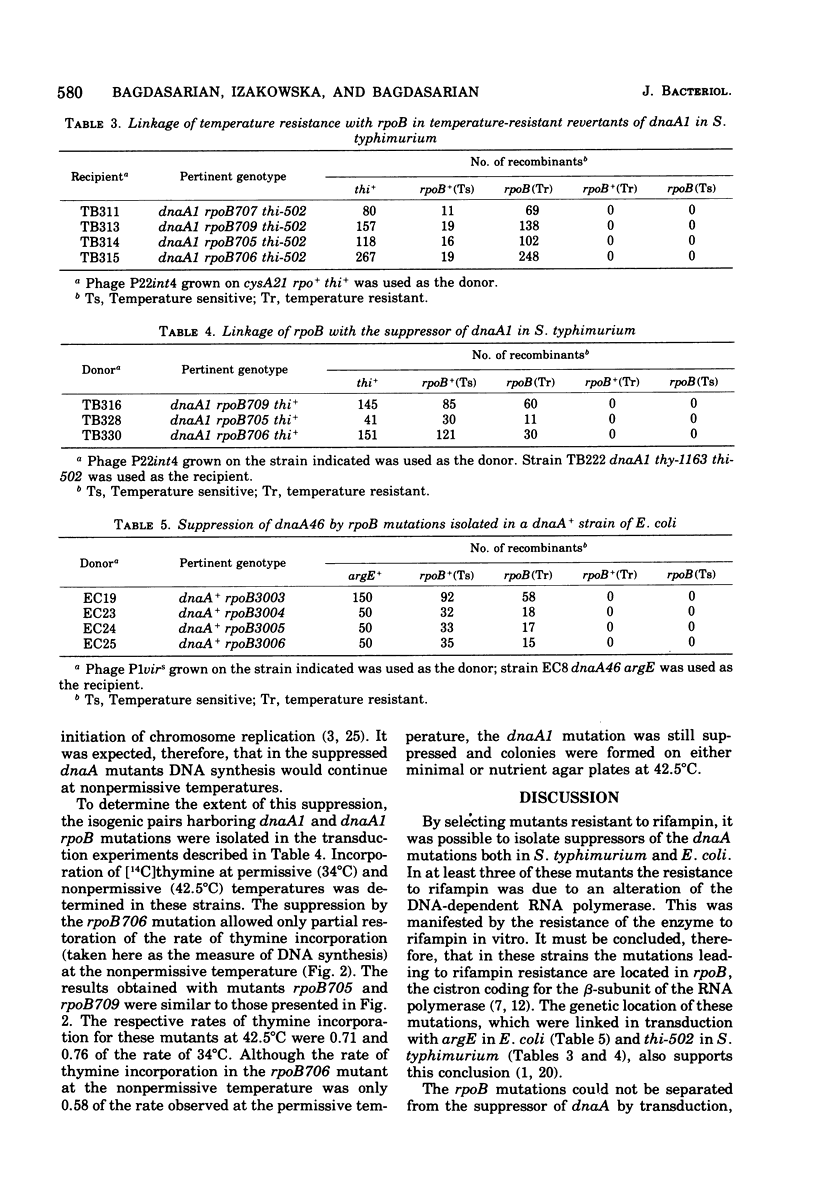
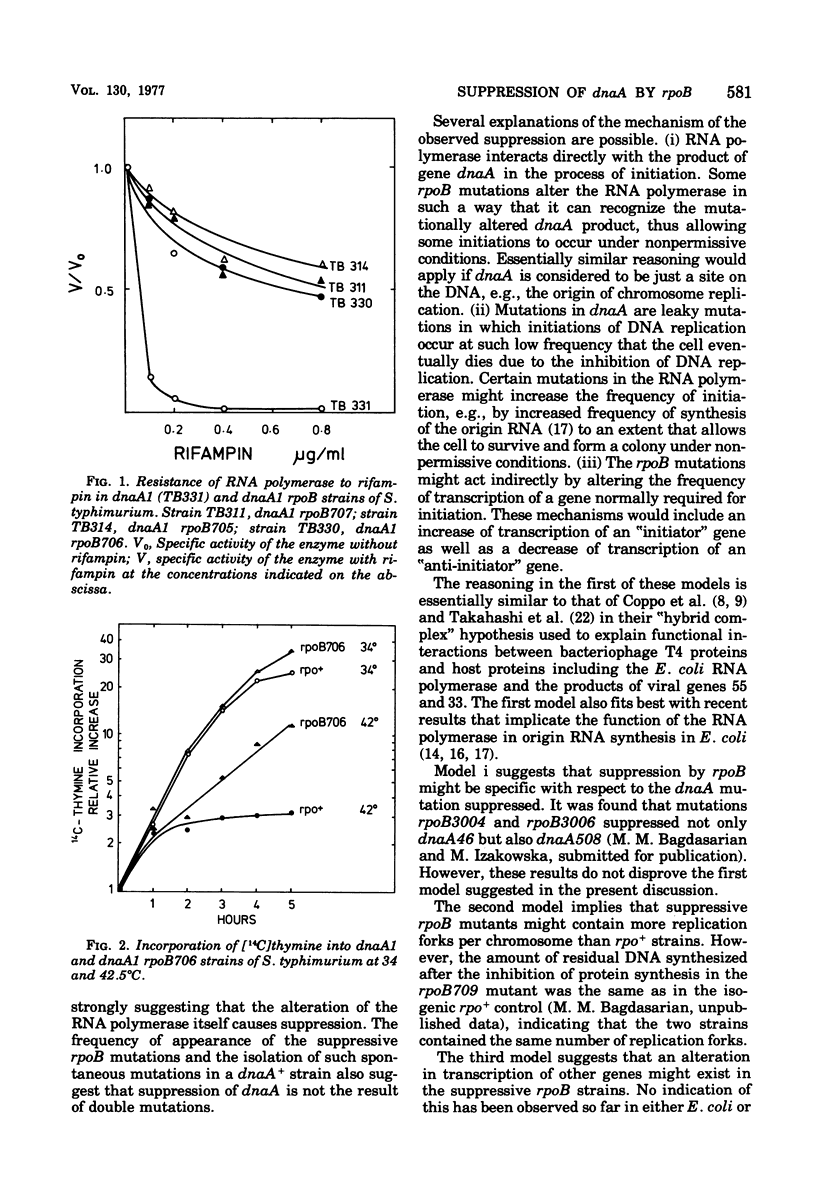
A class of mutations that confer resistance to rifampin in Salmonella typhimurium and Escherichia coli also suppresses the thermosensitivity of chromosome initiation in dnaA mutants. Ribonucleic acid polymerase is resistant to rifampin in vitro in these suppressive mutants, and the suppressors of dnaA cannot be separated from the rpoB mutations by transduction. It is concluded, therefore, that certain rpoB mutations may suppress the DnaA phenotype.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Hryniewicz M., Zdzienicka M. Integrative suppression of a dnaA mutation in Salmonella typhimurium. Mol Gen Genet. 1975 Aug 27;139(3):213–231. doi: 10.1007/BF00268973. [DOI] [PubMed] [Google Scholar]
- Barker S. T., Kurtz H., Taylor B. A., Ackermann W. W. A covalently linked DNA-RNA molecule from human leukemia cells. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1068–1074. doi: 10.1016/0006-291x(73)91515-5. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Chandler M., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R.100.1: Change of chromosome replication origin in synchronized cultures. J Bacteriol. 1976 Jun;126(3):1215–1223. doi: 10.1128/jb.126.3.1215-1223.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F. Host mutant (tabD)-induced inhibition of bacteriophage T4 late transcription. II. Genetic characterization of mutants. J Mol Biol. 1975 Aug 25;96(4):601–624. doi: 10.1016/0022-2836(75)90141-2. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Host mutant (tabD)-induced inhibition of bacteriophage T4 late transcription. I. Isolation and phenotypic characterization of the mutants. J Mol Biol. 1975 Aug 25;96(4):579–600. doi: 10.1016/0022-2836(75)90140-0. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Laurent S. J. Initiation of deoxyribonucleic acid replication in a temperature-sensitive mutant of B. subtilis: evidence for a transcriptional step. J Bacteriol. 1973 Oct;116(1):141–145. doi: 10.1128/jb.116.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Coppo A., Manzi A., Martire G., Pulitzer J. F. Design of a system of conditional lethal mutations (tab/k/com) affecting protein-protein interactions in bacteriophage T4-infected Escherichia coli. J Mol Biol. 1975 Aug 25;96(4):563–578. doi: 10.1016/0022-2836(75)90139-4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Waqar M. A., Huberman J. A. Evidence for the attachment of RNA to pulse-labeled DNA in the slime mold, Physarum polycephalum. Biochem Biophys Res Commun. 1973 Mar 5;51(1):174–180. doi: 10.1016/0006-291x(73)90524-x. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



