Abstract
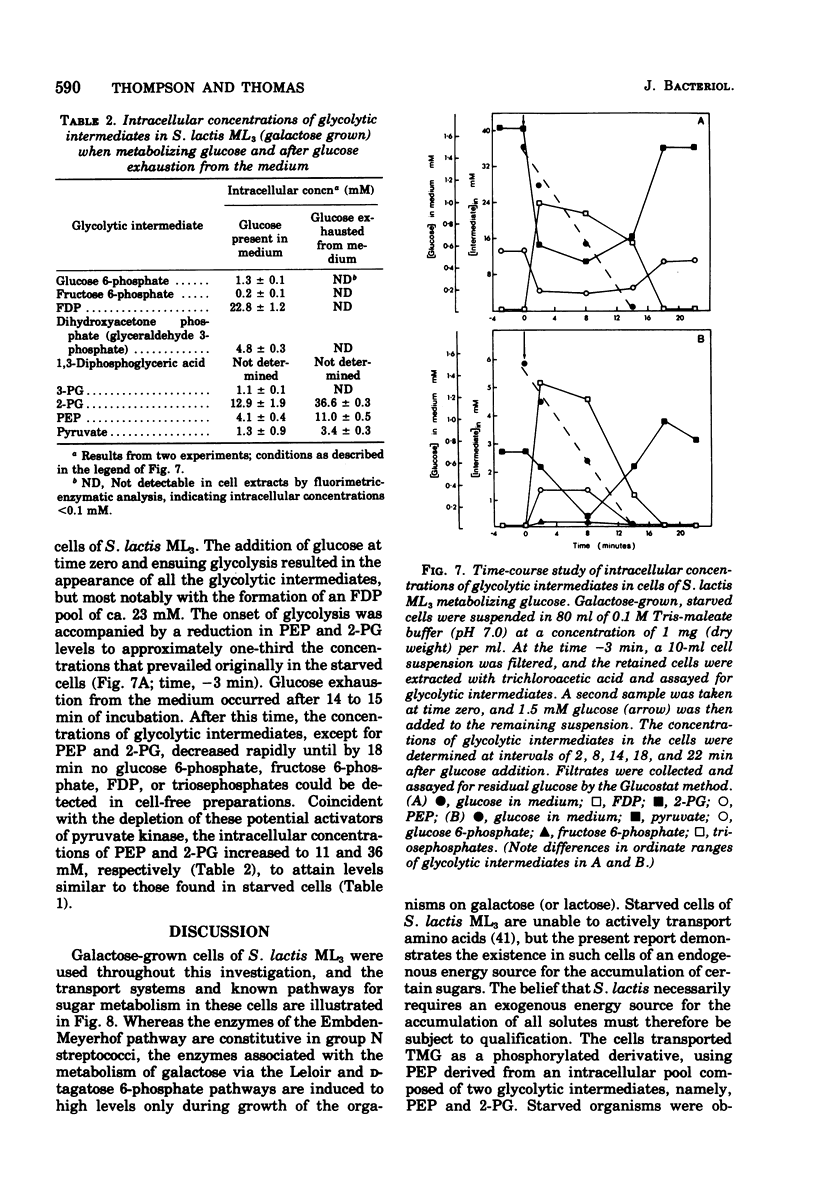
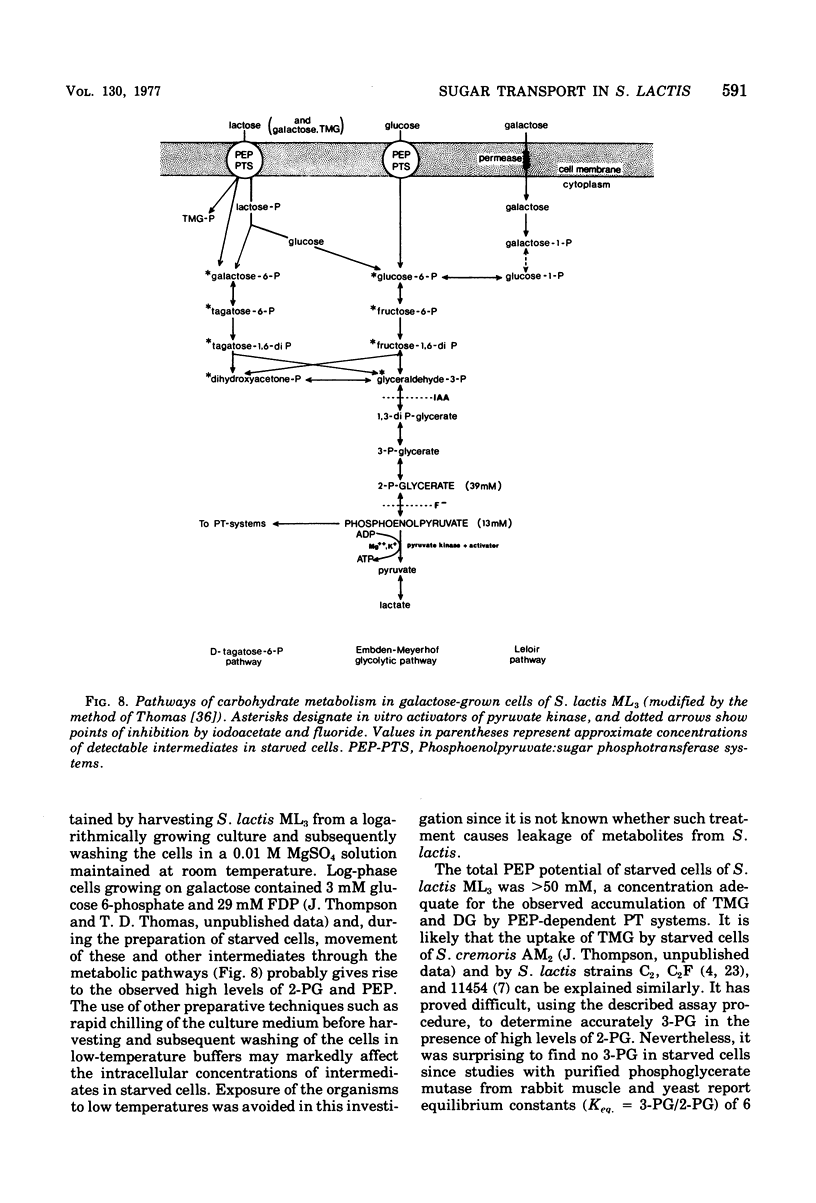
In the absence of an exogenous energy source, galactose-grown cells of Streptococcus lactis ML3 rapidly accumulated thiomethyl-beta-D-galactopyranoside (TMG) and 2-deoxyglucose to intracellular concentrations of 40 to 50 mM. Starved cells maintained the capacity for TMG uptake for many hours, and accumulation of the beta-galactoside was insensitive to proton-conducting ionophores (tetrachlorosalicylanilide and carbonylcyanide-m-chlorophenyl hydrazone) and sulfydryl group reagents including iodoacetate and N-ethylmaleimide. Fluorimetric analysis of glycolytic intermediates in extracts prepared from starved cells revealed (a) high intracellular levels of phosphoenolpyruvate (13 mM; PEP) and 2-phosphoglycerate (approximately 39 mM; 2-PG), but an absence of other metabolites including glucose 6-phosphate, fructose 6-phosphate, fructose 1,6-diphosphate, and triosephosphates. The following criteria showed PEP (and 2-PG) to be the endogenous energy source for TMG accumulation by the phosphotransferase system: the intracellular concentrations of PEP and 2-PG decreased with concomitant uptake of TMG, and a close correlation was observed between maximum accumulation of the beta-galactoside and the total available concentration of the two intermediates; TMG accumulated as an anionic derivative, which after extraction and incubation with alkaline phosphatase (EC 3.1.3.1) formed the original analogue; fluoride inhibition of 2-phospho-D-glycerate hydrolyase (EC 4.2.1.11) prevented the conversion of 2-PG to PEP, and uptake of TMG by the starved cells was reduced by 80%; and the stoichiometric ratio [TMG] accumulated/[PEP] consumed was almost unity (0.93). In cells metabolizing glucose, all intermediates listed in (a) and (b) were found. Upon exhaustion of glucose from the medium, the metabolites in (b) were not longer detectable, while the intracellular concentrations of PEP and 2-PG increased to the levels previously observed in starved cells. The glycolytic intermediates in (b) are all in vitro heterotropic effectors of pyruvate kinase (adenosine 5'-triphosphate:pyruvate 2-O-phosphotransferase, EC 2.7.1.40) from S. lactis ML3. It is suggested that the capacity of starved cells to maintain high intracellular concentrations of PEP and 2-PG is a consequence of decreased in vivo activity of this key regulatory enzyme of glycolysis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L. Characterization of lactose-fermenting revertants from lactose-negative Streptococcus lactis C2 mutants. J Bacteriol. 1974 Sep;119(3):830–839. doi: 10.1128/jb.119.3.830-839.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Purification and properties of pyruvate kinase from Streptococcus lactis. Biochim Biophys Acta. 1976 Jun 7;438(1):90–101. doi: 10.1016/0005-2744(76)90225-4. [DOI] [PubMed] [Google Scholar]
- Demko G. M., Blanton S. J., Benoit R. E. Heterofermentative carbohydrate metabolism of lactose-impaired mutants of Streptococcus lactis. J Bacteriol. 1972 Dec;112(3):1335–1345. doi: 10.1128/jb.112.3.1335-1345.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Studies with fluoride-sensitive and fluoride-resistant strains of Streptococcus salivarius. II. Fluoride inhibition of glucose metabolism. Can J Microbiol. 1969 Sep;15(9):1021–1027. doi: 10.1139/m69-182. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Chemiosmotic interpretation of active transport in bacteria. Ann N Y Acad Sci. 1974 Feb 18;227:297–311. doi: 10.1111/j.1749-6632.1974.tb14395.x. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. T., Utech N. M., Reid K. G. Alpha-methyl-L-glutamic acid uptake by high affinity dicarboxylic amino acid transport system in Streptococcus faecalis. Biochim Biophys Acta. 1975 Jun 11;394(1):55–64. doi: 10.1016/0005-2736(75)90204-7. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Galactoside accumulation associated with ion movements in Streptococcus lactis. Biochem Biophys Res Commun. 1972 Nov 1;49(3):615–620. doi: 10.1016/0006-291x(72)90455-x. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Protonmotive force in fermenting Streptococcus lactis 7962 in relation to sugar accumulation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):879–886. doi: 10.1016/s0006-291x(74)80061-6. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue P., MacDonald R. E. Identification of thiomethyl-beta-D-galactoside 6-phosphate accumulated by Staphylococcus aureus. J Biol Chem. 1968 Feb 10;243(3):680–682. [PubMed] [Google Scholar]
- Lee R., Molskness T., Sandine W. E., Elliker P. R. Carbohydrate metabolism in lactic streptococci: fate of galactose supplied in free or disaccharide form. Appl Microbiol. 1973 Dec;26(6):951–958. doi: 10.1128/am.26.6.951-958.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- MAITRA P. K., ESTABROOK R. W. A FLUOROMETRIC METHOD FOR THE ENZYMIC DETERMINATION OF GLYCOLYTIC INTERMEDIATES. Anal Biochem. 1964 Apr;7:472–484. doi: 10.1016/0003-2697(64)90156-3. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. J Bioenerg. 1972 May;3(1):5–24. doi: 10.1007/BF01515993. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Ciba Found Symp. 1975;(31):225–241. doi: 10.1002/9780470720134.ch13. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Thomas T. D. Activator specificity of pyruvate kinase from lactic streptococci. J Bacteriol. 1976 Mar;125(3):1240–1242. doi: 10.1128/jb.125.3.1240-1242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Metabolism of exogenous arginine and glucose by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):371–380. doi: 10.1099/00221287-58-3-371. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Survival of Streptococcus lactis in starvation conditions. J Gen Microbiol. 1968 Mar;50(3):367–382. doi: 10.1099/00221287-50-3-367. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Synthesis of protein and ribonucleic acid by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):363–369. doi: 10.1099/00221287-58-3-363. [DOI] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]