Abstract
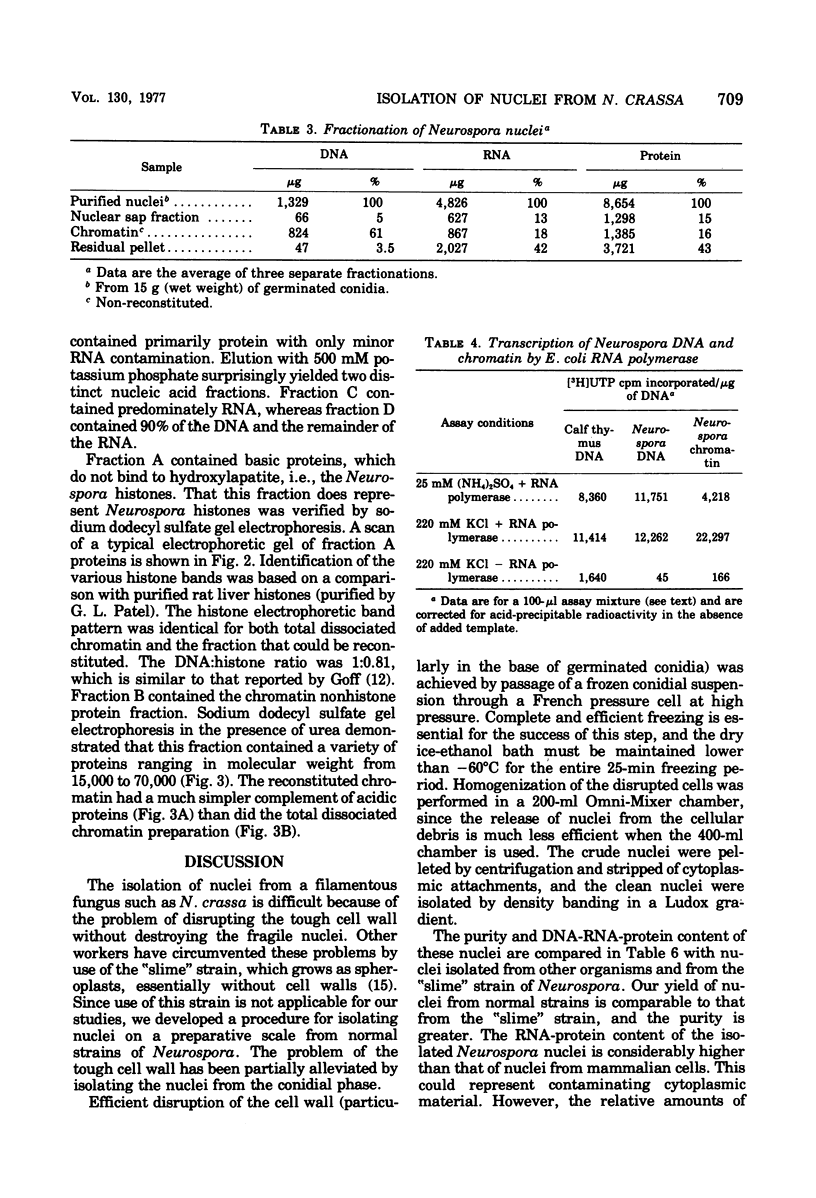
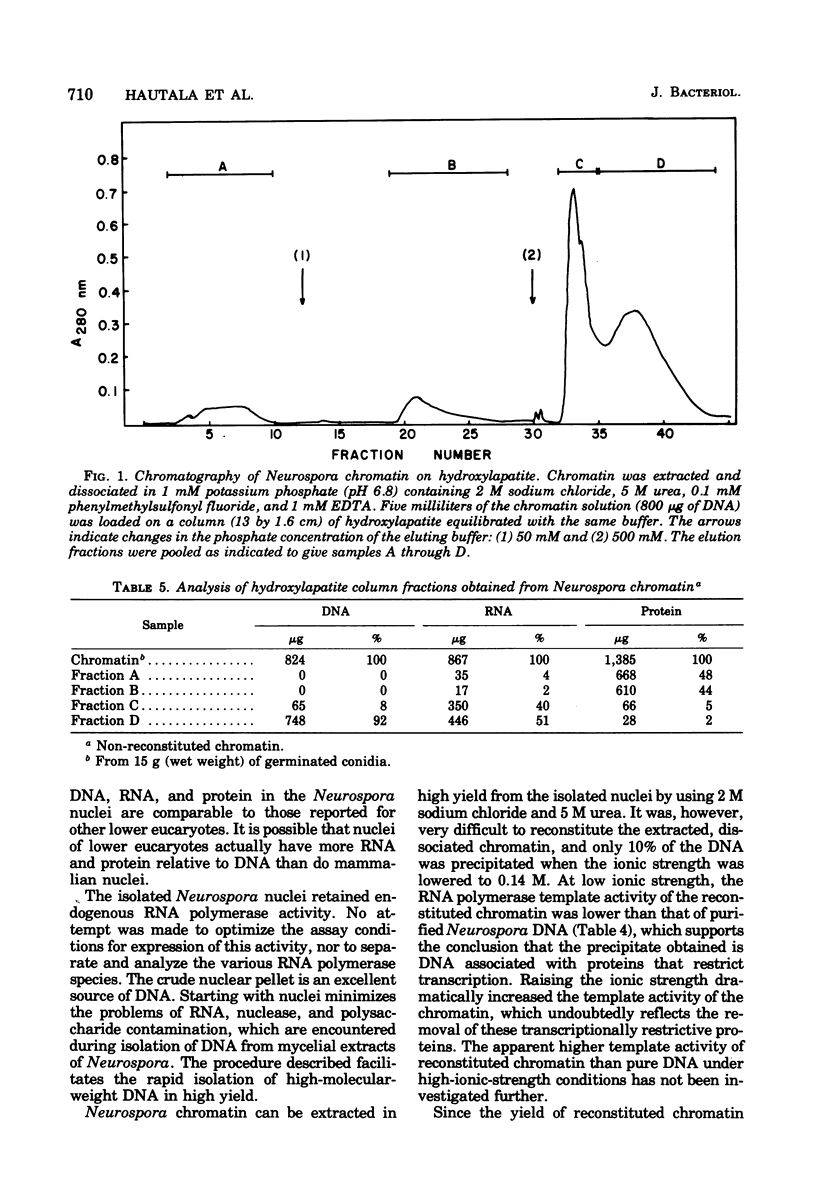
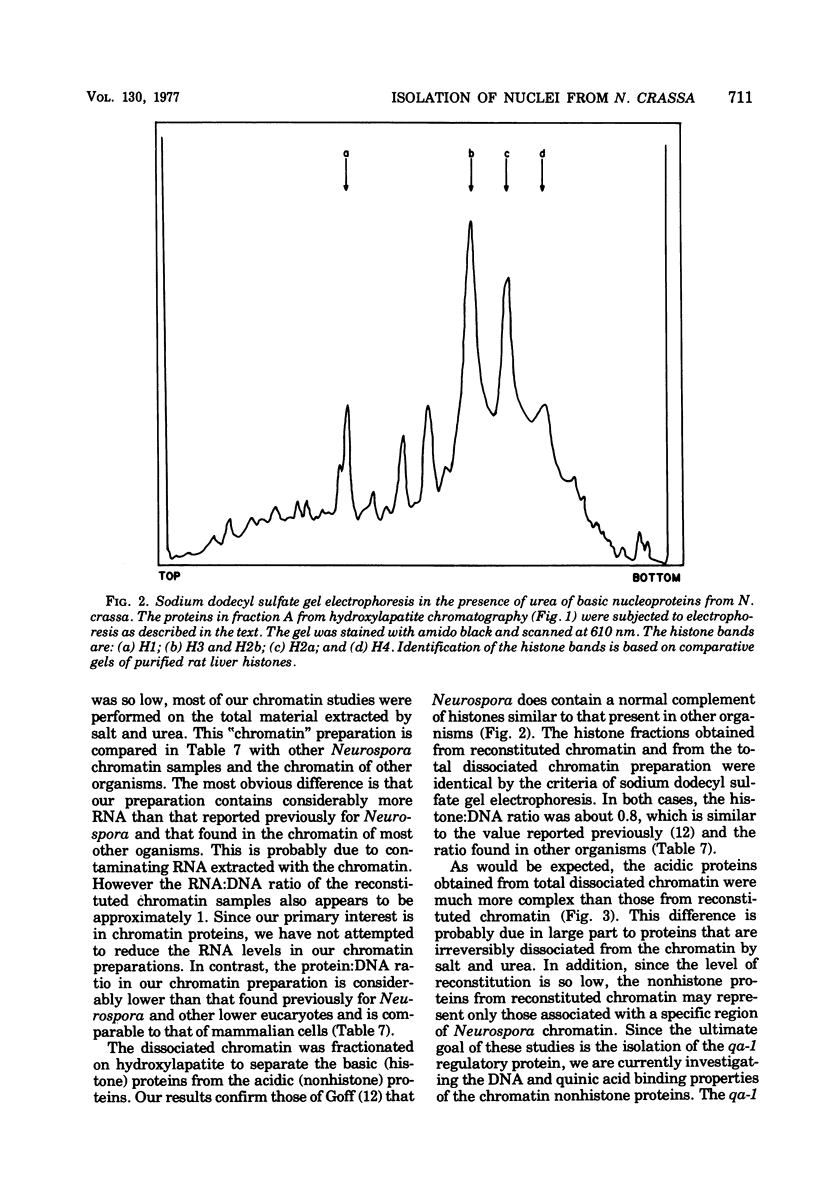
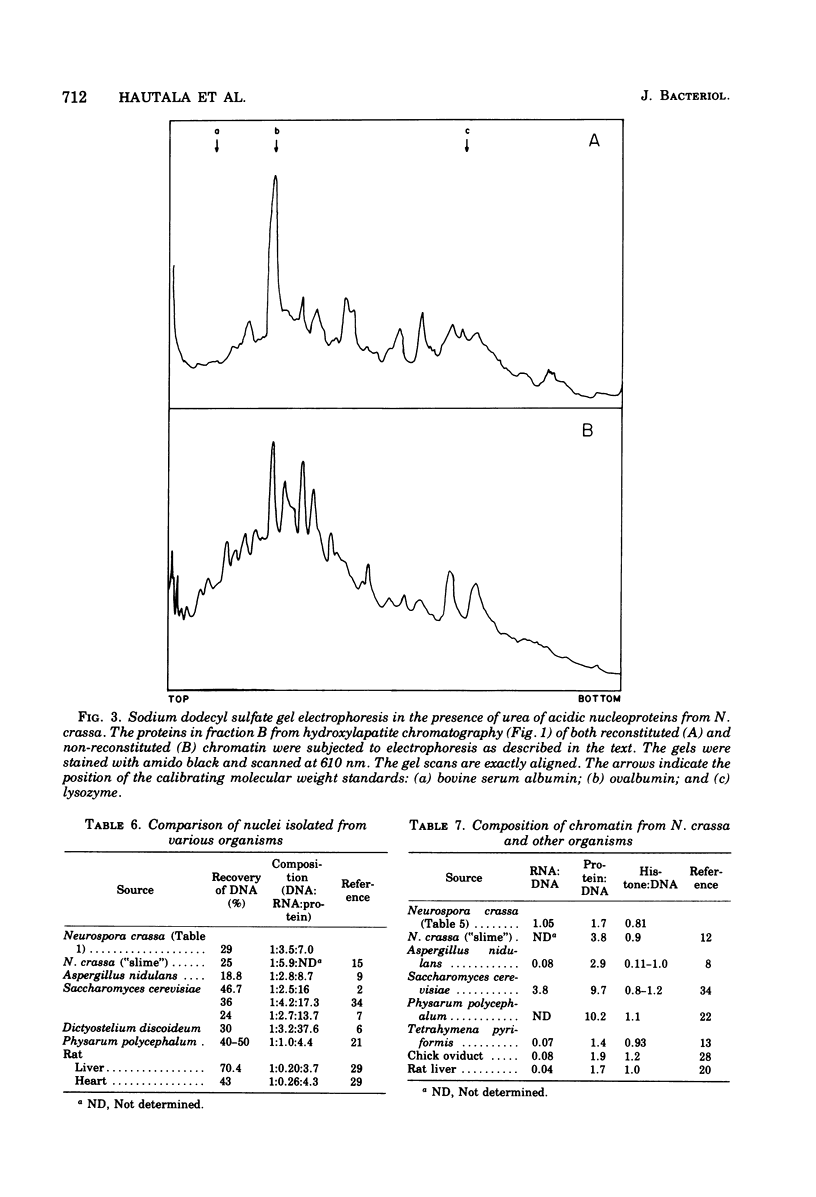
A procedure was developed for isolating nuclei from either the conidial or germinated conidial growth phase of Neurospora crassa. A frozen conidial suspension was lysed by passage through a French pressure cell, and the nuclei were freed from the broken cells by repeated homogenization in an Omni-Mixer. Pure nuclei were obtained from the crude nuclear fraction by density banding in a Ludox gradient. The final nuclear yield was 20 to 30%. The nuclei had a deoxyribonucleic acid (DNA):ribonucleic acid (RNA):protein ratio of 1:3.5:7 and were active in RNA synthesis. The nuclei, stained with the DNA stain 4,6-diamidino-2-phenylindole, appeared under fluorescence microscopy as bright blue spheres, 1 micron in diameter, essentially free from cytoplasmic attachments. Chromatin extracted from the nuclei in a 70 to 75% yield by dissociation with 2 M sodium chloride and 5 M urea had a DNA:RNA:protein ratio of 1:1.05:1.7. Chromatin reconstituted from this preparation exhibited a level of RNA polymerase template activity lower than that of pure Neurospora DNA, but the maximum level of reconstitution obtained was only 10%. Fractionation of Neurospora chromatin on hydroxylapatite separated the histones from the chromatin acidic proteins. The normal complement of histone proteins was present in both the reconstituted and dissociated chromatin preparations. The acidic protein fraction exhibited a variety of bands on sodium dodecyl sulfate gel electrophoresis ranging in molecular weight from 15,000 to 70,000. The gel pattern was much more complex for total dissociated chromatin than for reconstituted chromatin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekhor I., Kung G. M., Bonner J. Sequence-specific interaction of DNA and chromosomal protein. J Mol Biol. 1969 Jan;39(2):351–364. doi: 10.1016/0022-2836(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Brooks R. R., Huang P. C. Redundant DNA of Neurospora crassa. Biochem Genet. 1972 Feb;6(1):41–49. doi: 10.1007/BF00485964. [DOI] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Genetic evidence on the organization and action of the qa-1 gene product: a protein regulating the induction of three enzymes in quinate catabolism in Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Feb;72(2):553–557. doi: 10.1073/pnas.72.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleff R. S. The inducible quinate-shikimate catabolic pathway in Neurospora crassa: genetic organization. J Gen Microbiol. 1974 Apr;81(2):337–355. doi: 10.1099/00221287-81-2-337. [DOI] [PubMed] [Google Scholar]
- Charlesworth M. C., Parish R. W. The isolation of nuclei and basic nucleoproteins from the cellular slime mold Dictyostelium discoideum. Eur J Biochem. 1975 May;54(1):307–316. doi: 10.1111/j.1432-1033.1975.tb04141.x. [DOI] [PubMed] [Google Scholar]
- Doi K., Doi A. Isolation of nuclei from a tetraploid strain of Saccharomyces cerevisiae. J Biochem. 1974 May;75(5):1017–1026. doi: 10.1093/oxfordjournals.jbchem.a130473. [DOI] [PubMed] [Google Scholar]
- Felden R. A., Sanders M. M., Morris N. R. Presence of histones in Aspergillus nidulans. J Cell Biol. 1976 Mar;68(3):430–439. doi: 10.1083/jcb.68.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealt M. A., Sheir-Neiss G., Morris N. R. The isolation of nuclei from the filamentous fungus Aspergillus nidulans. J Gen Microbiol. 1976 May;94(1):204–210. doi: 10.1099/00221287-94-1-204. [DOI] [PubMed] [Google Scholar]
- Goff C. G. Histones of Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):4131–4138. [PubMed] [Google Scholar]
- Hamana K., Iwai K. Fractionation and characterization of Tetrahymena histone in comparison with mammalian histones. J Biochem. 1971 Jun;69(6):1097–1111. doi: 10.1093/oxfordjournals.jbchem.a129563. [DOI] [PubMed] [Google Scholar]
- Hsiang M. W., Cole R. D. The isolation of histone from Neurospora crassa. J Biol Chem. 1973 Mar 25;248(6):2007–2013. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lurquin P. F., Tshitenge G., Delaunoit G., Ledoux L. Isolation of DNA from plant cells by gel filtration on agarose. Anal Biochem. 1975 May 12;65(1-2):1–4. doi: 10.1016/0003-2697(75)90483-2. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MacGillivray A. J., Cameron A., Krauze R. J., Rickwood D., Paul J. The non-histone proteins of chromatin, their isolation and composition in a number of tissues. Biochim Biophys Acta. 1972 Aug 25;277(2):384–402. [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation and DNA content of nuclei of Physarum polycephalum. Exp Cell Res. 1971 Jun;66(2):305–316. doi: 10.1016/0014-4827(71)90682-3. [DOI] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Nuclear histones in Physarum polycephalum during growth and differentiation. Arch Biochem Biophys. 1970 Jun;138(2):418–432. doi: 10.1016/0003-9861(70)90365-6. [DOI] [PubMed] [Google Scholar]
- Patel G. L. Chromatin acidic proteins with high affinity for nucleohistone and DNA. Life Sci II. 1972 Dec 8;11(23):1135–1142. doi: 10.1016/0024-3205(72)90268-8. [DOI] [PubMed] [Google Scholar]
- REICH E., TSUDA S. Isolation of nuclei of Neurospora crassa. Biochim Biophys Acta. 1961 Nov 11;53:574–575. doi: 10.1016/0006-3002(61)90217-7. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska E. M., Bhargava M. M., Arnaud M. V., Halvorson H. O. An improved method for the isolation of yeast nuclei active in RNA synthesis in vitro. Biochem Biophys Res Commun. 1974 Jan 23;56(2):496–502. doi: 10.1016/0006-291x(74)90870-5. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Knowler J. T., Moses H. L. Specific methods for the isolation of nuclei from chick oviduct. Methods Enzymol. 1974;31:263–279. doi: 10.1016/0076-6879(74)31028-2. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Isolation of nuclei from liver and other tissues. Methods Enzymol. 1974;31:253–262. doi: 10.1016/0076-6879(74)31027-0. [DOI] [PubMed] [Google Scholar]
- Valone J. A., Jr, Case M. E., Giles N. H. Constitutive mutants in a regulatory gene exerting positive control of quinic acid catabolism in Neurospora crassa. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1555–1559. doi: 10.1073/pnas.68.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/s0091-679x(08)60963-2. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Smith P., Letnansky K. Yeast chromatin. Preparation from isolated nuclei, histone composition and transcription capacity. Eur J Biochem. 1973 Feb 15;33(1):123–130. doi: 10.1111/j.1432-1033.1973.tb02663.x. [DOI] [PubMed] [Google Scholar]



