Abstract
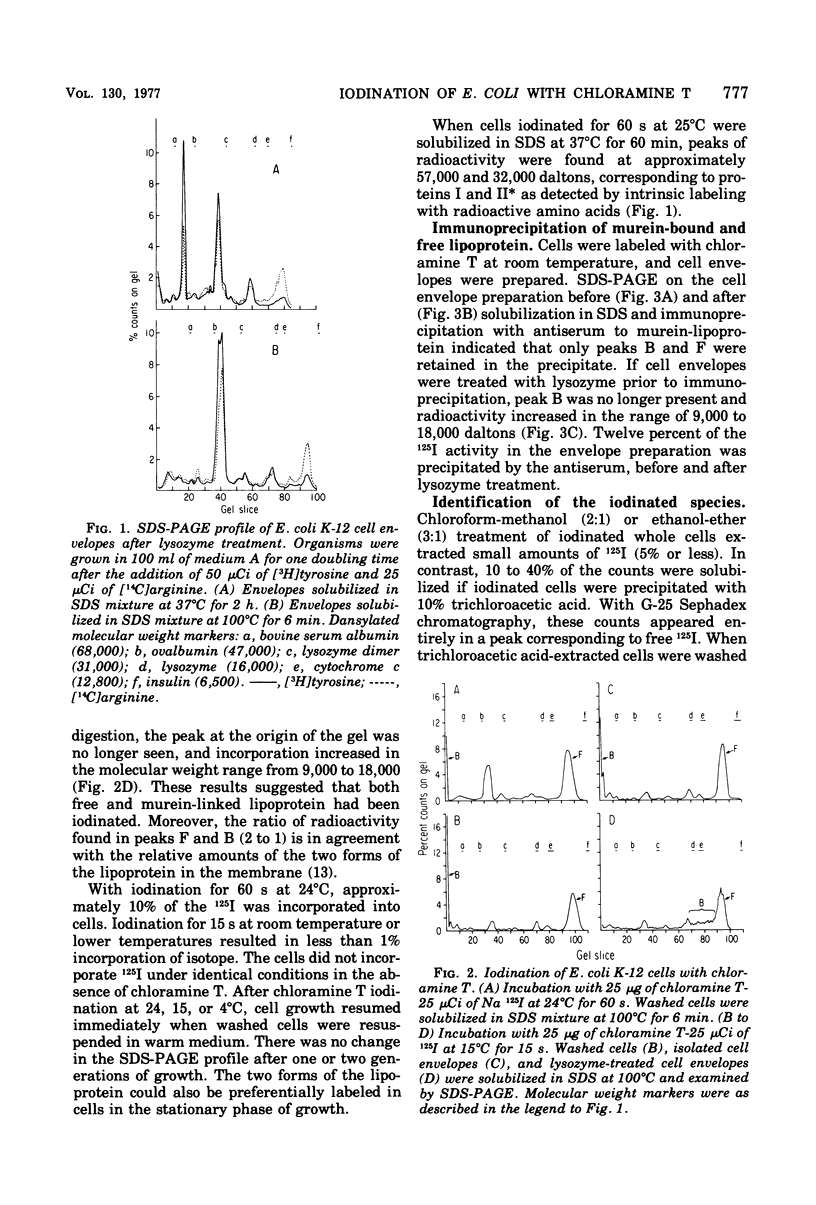
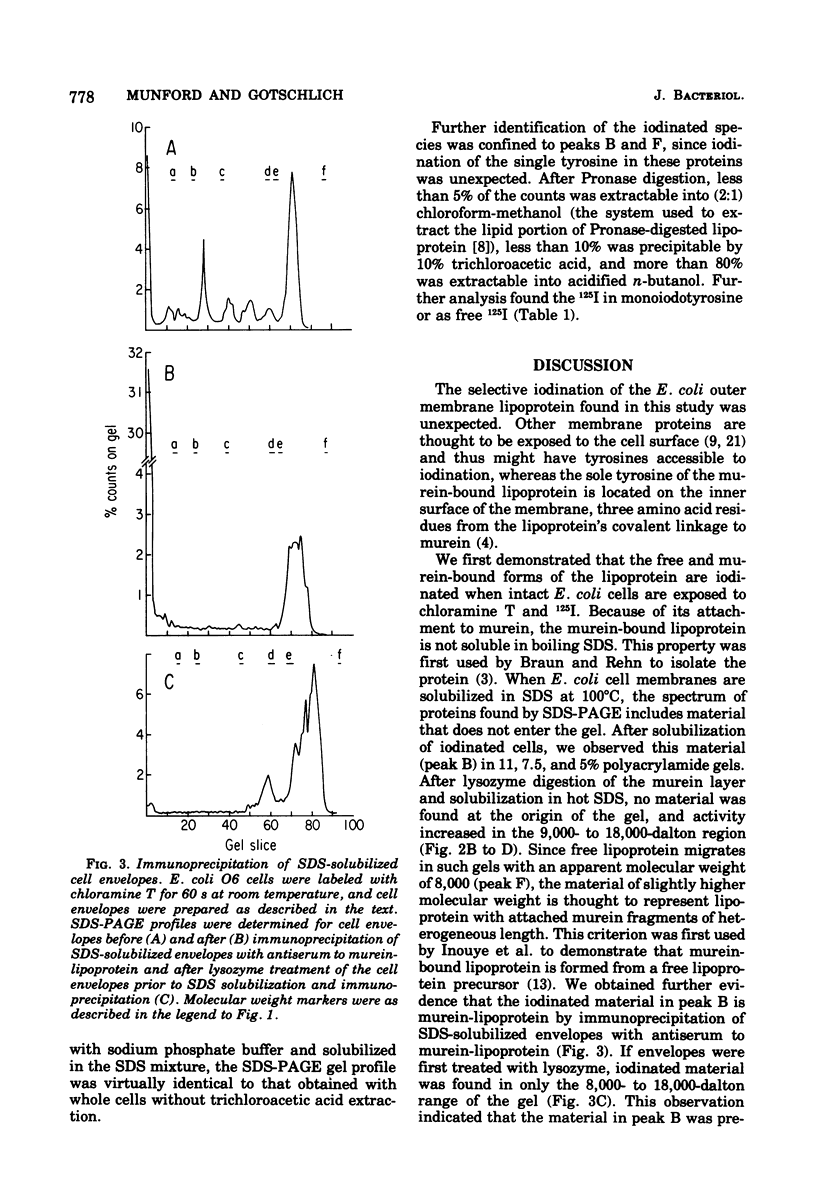
Iodination of Escherichia coli cells with chloramine T preferentially labels the free and murein-bound forms of the outer membrane lipoprotein. Iodination for 15 s at 15 degrees C labels the two forms of the lipoprotein almost exclusively, whereas iodination for 60 s at 25 degrees C also labels the other major outer membrane proteins. Chloramine T iodination is a rapid, simple technique for labeling the outer membrane lipoprotein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Hantke K. Characterization of the free form of murein-lipoprotein from the outer membrane of Escherichia coli B/r. FEBS Lett. 1975 Dec 1;60(1):26–28. doi: 10.1016/0014-5793(75)80410-8. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Hirashima A., Inouye M. Existence of a free form of a specific membrane lipoprotein in gram-negative bacteria. J Bacteriol. 1974 Dec;120(3):1204–1208. doi: 10.1128/jb.120.3.1204-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller I., Hoehn B., Henning U. Apparent high degree of asymmetry of protein arrangement in the Escherichia coli outer cell envelope membrane. Biochemistry. 1975 Feb 11;14(3):478–484. doi: 10.1021/bi00674a003. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Haller I. Mutants of Escherichia coli K12 lacking all 'major' proteins of the outer cell envelope membrane. FEBS Lett. 1975 Jul 15;55(1):161–164. doi: 10.1016/0014-5793(75)80983-5. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Homogeneity of envelope proteins of Escherichia coli separated by gel electrophoresis in sodium dodecyl sulfate. J Bacteriol. 1973 Jan;113(1):304–312. doi: 10.1128/jb.113.1.304-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo J. L., Roncone A., Izzo M. J., Foley R., Bartlett J. W. Degradation of 131 I-insulins by rat liver. Studies in vitro. J Biol Chem. 1972 Feb 25;247(4):1219–1226. [PubMed] [Google Scholar]
- James R., Gudas L. J. Cell cycle-specific incorporation of lipoprotein into the outer membrane of Escherichia coli. J Bacteriol. 1976 Jan;125(1):374–375. doi: 10.1128/jb.125.1.374-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- ROCHE J., LISSITZKY S., MICHEL R. Chromatographic analysis of radioactive iodine compounds from the thyroid gland and body fluids. Methods Biochem Anal. 1954;1:243–264. doi: 10.1002/9780470110171.ch9. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


