Abstract
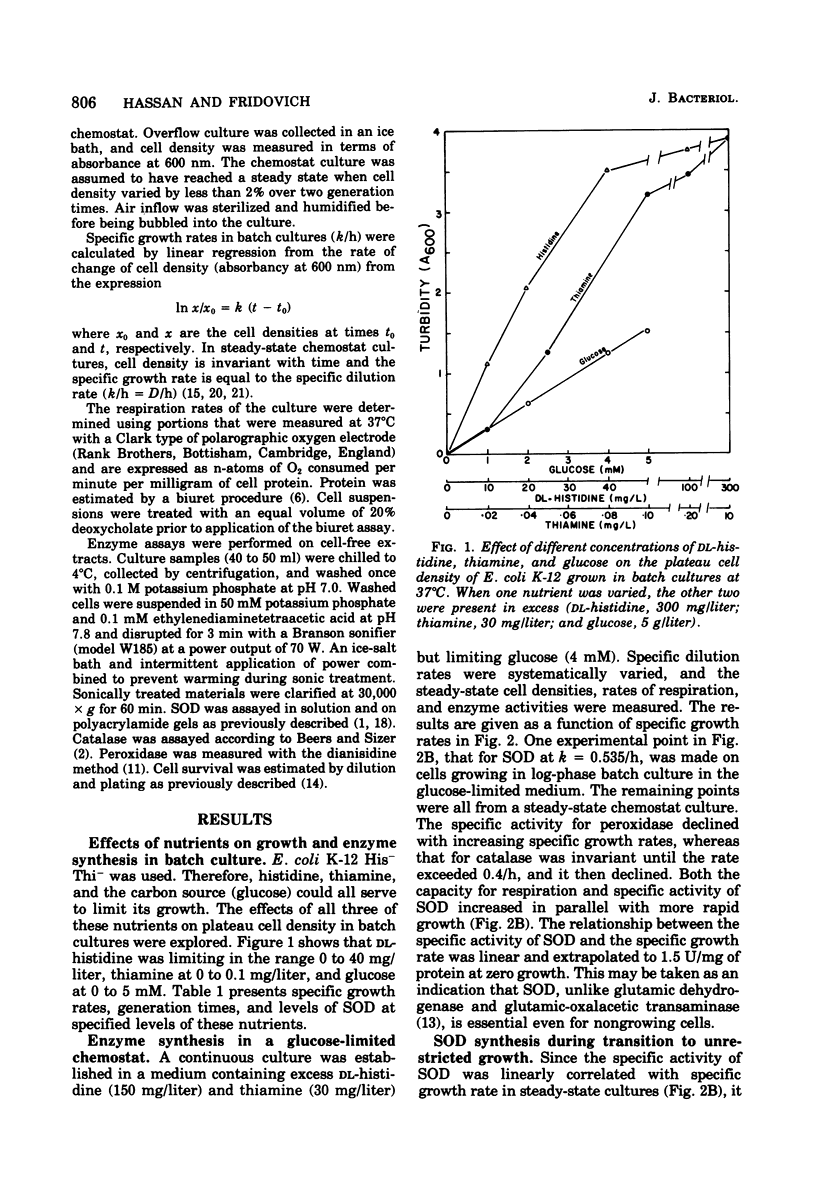
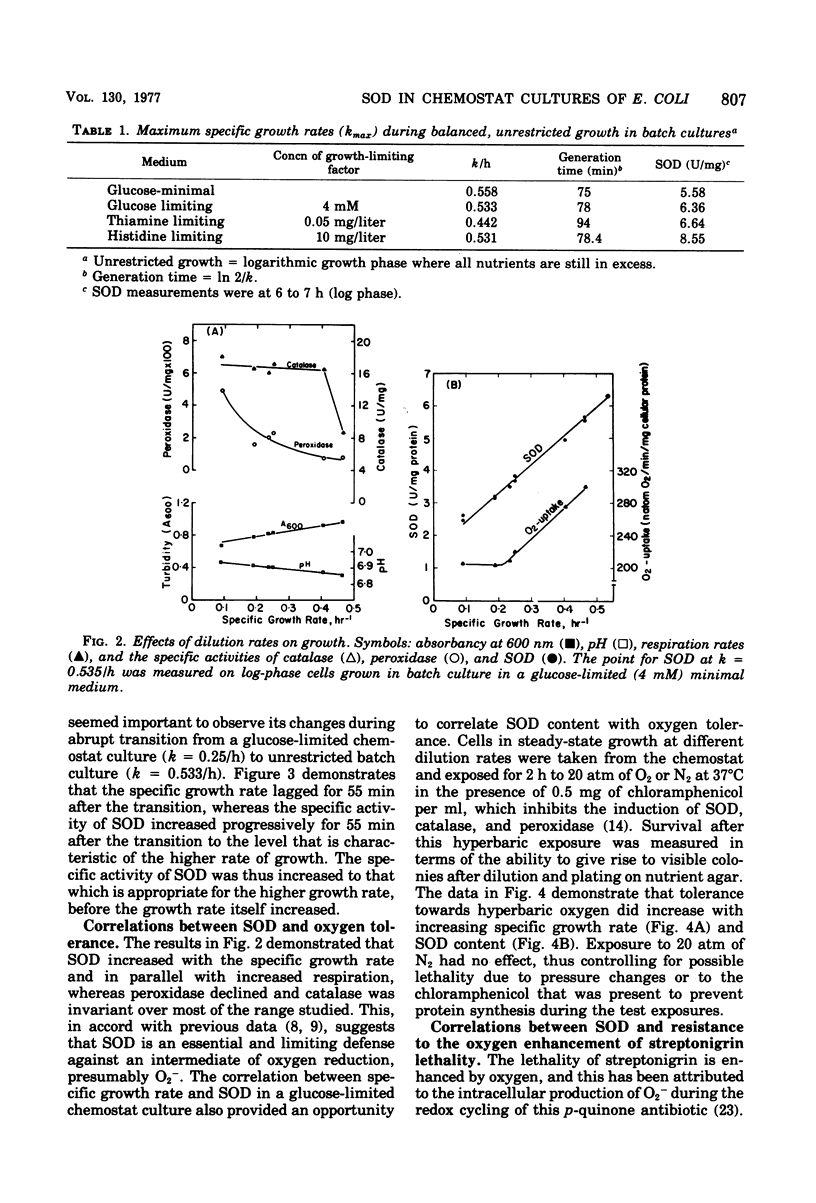
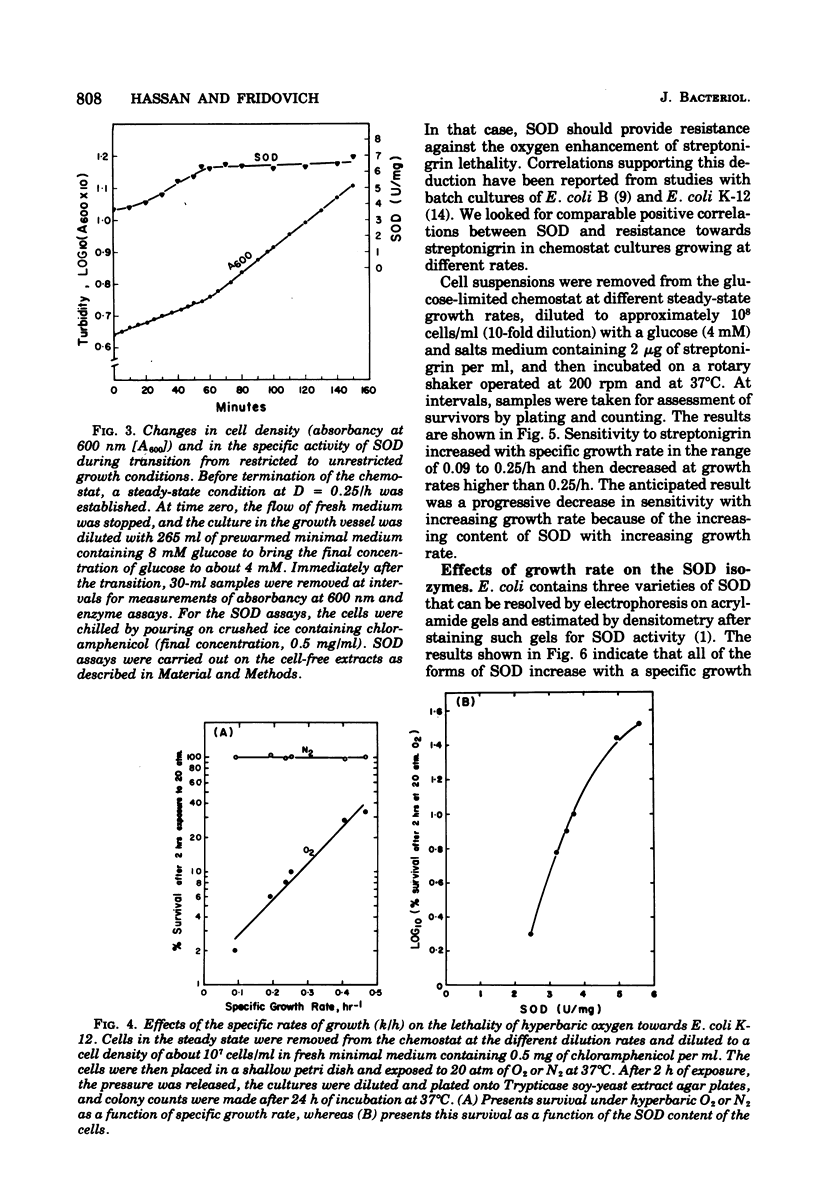
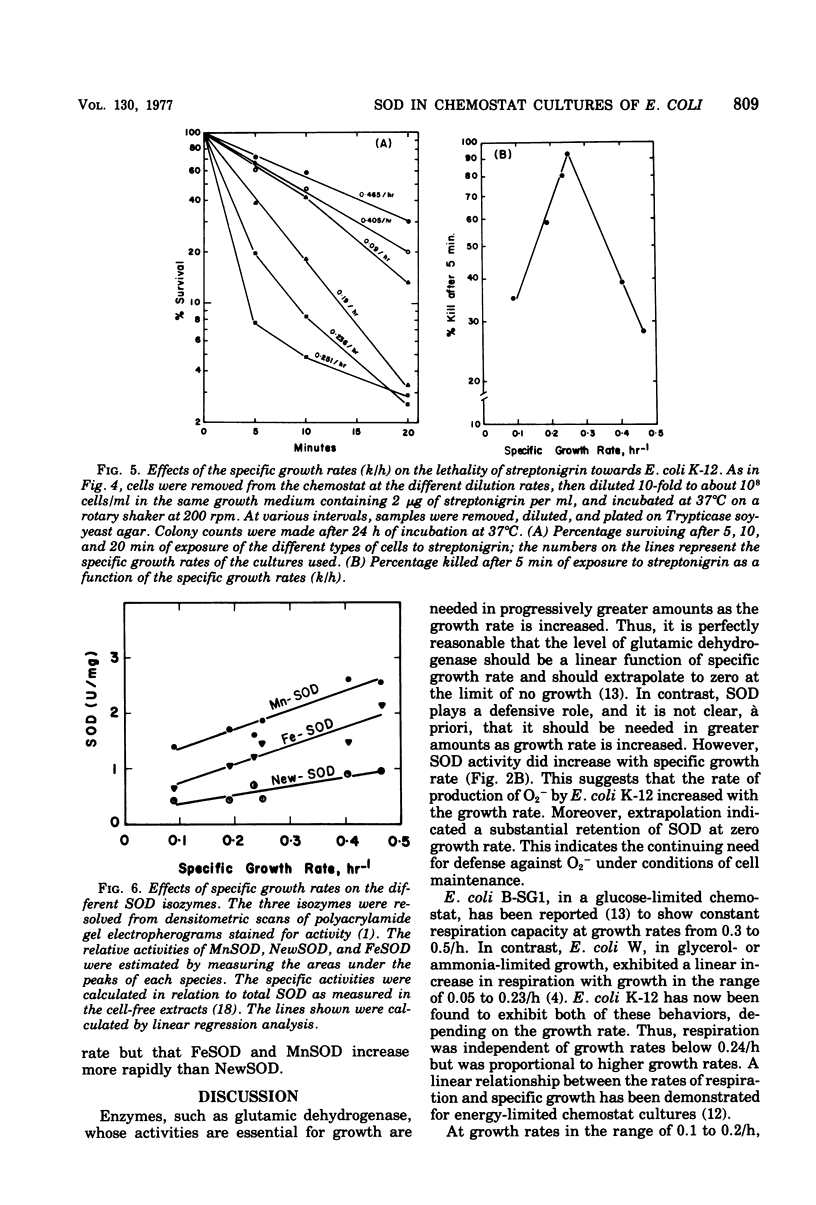
Conditions for continuous culture of Escherichia coli K-12 His- Thi- under glucose limitation were established. Both the capacity for respiration, at D greater than 0.2/h, and specific activity of superoxide dismutase increased as a function of specific growth rate, whereas peroxidase and catalase were either invariant with or inversely related to this growth rate. The abrupt increase in the availability of glucose, as a means of elevating the growth rate, was followed by an increase in superoxide dismutase, which reached a plateau before there was a significant increase in the growth rate. Thus, an increase in superoxide dismutase appeared to be a prerequisite for an increase in the rate of growth. Cells that had higher levels of superoxide dismutase, because of varying specific growth rates, were more resistant to the toxicity of hyperbaric oxygen. Superoxide dismutase thus behaved like an essential defense against the toxicity of oxygen. Sensitivity towards streptonigrin increased with specific growth rate in the range of 0.09 to 0.25/h but decreased with further increases in the growth rate. Since this antibiotic has been shown to shunt electrons to oxygen, with concomitant production of O2-, these results indicated a progressive deficiency of reducing power at growth rates below 0.25/h and a surfeit of reducing power with progressively greater protection against O2- by superoxide dismutase at growth rates greater than 0.25/h.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Cone R., Hasan S. K., Lown J. W., Morgan A. R. The mechanism of the degradation of DNA by streptonigrin. Can J Biochem. 1976 Mar;54(3):219–223. doi: 10.1139/o76-034. [DOI] [PubMed] [Google Scholar]
- Farmer I. S., Jones C. W. The energetics of Escherichia coli during aerobic growth in continuous culture. Eur J Biochem. 1976 Aug 1;67(1):115–122. doi: 10.1111/j.1432-1033.1976.tb10639.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Gottlieb S. F. Effect of hyperbaric oxygen on microorganisms. Annu Rev Microbiol. 1971;25:111–152. doi: 10.1146/annurev.mi.25.100171.000551. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Harvey R. J. Metabolic regulation in glucose-limited chemostat cultures of Escherichia coli. J Bacteriol. 1970 Nov;104(2):698–706. doi: 10.1128/jb.104.2.698-706.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugaard N. Cellular mechanisms of oxygen toxicity. Physiol Rev. 1968 Apr;48(2):311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- KJELDGAARD N. O., MAALOE O., SCHAECHTER M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):607–616. doi: 10.1099/00221287-19-3-607. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Description of the chemostat. Science. 1950 Dec 15;112(2920):715–716. doi: 10.1126/science.112.2920.715. [DOI] [PubMed] [Google Scholar]
- Swartz H. M. Toxic oxygen effects. Int Rev Cytol. 1973;35:321–343. doi: 10.1016/s0074-7696(08)60358-7. [DOI] [PubMed] [Google Scholar]
- White H. L., White J. R. Lethal action and metabolic effects of streptonigrin on Escherichia coli. Mol Pharmacol. 1968 Nov;4(6):549–565. [PubMed] [Google Scholar]



