Abstract
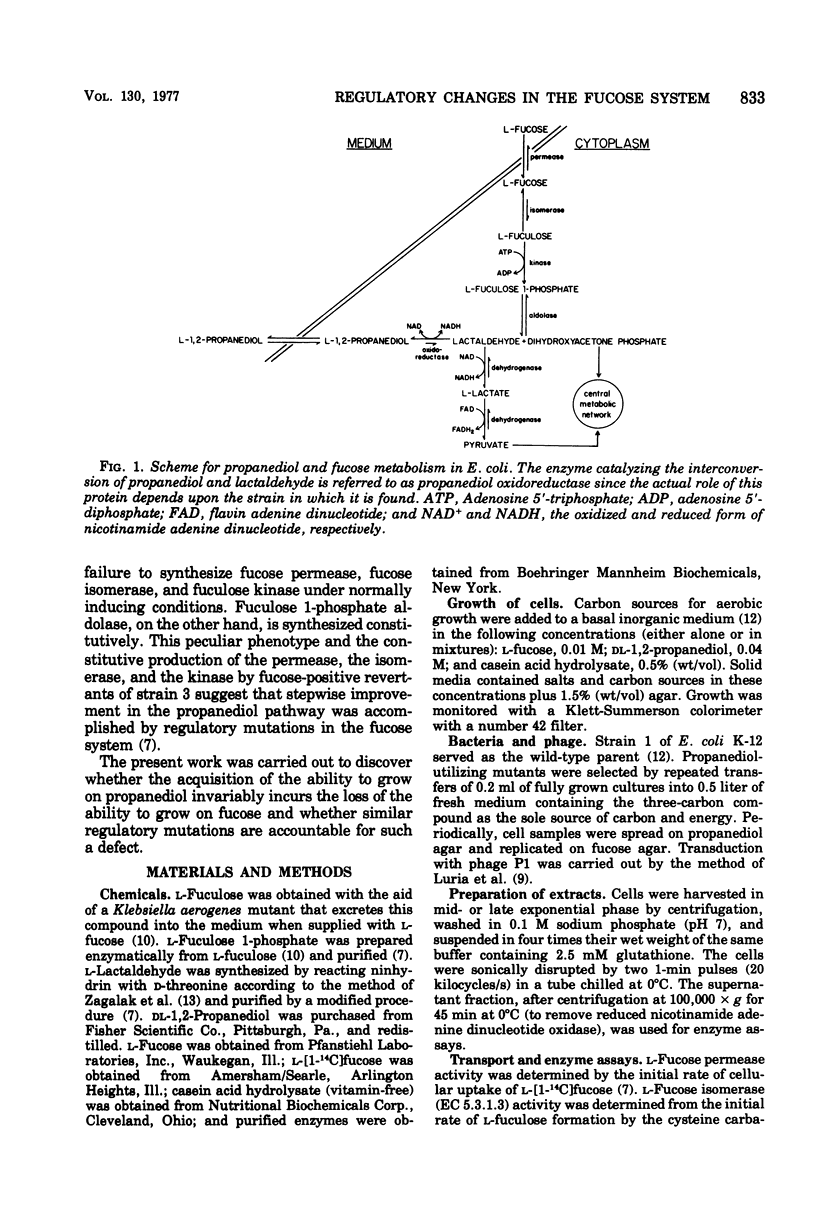
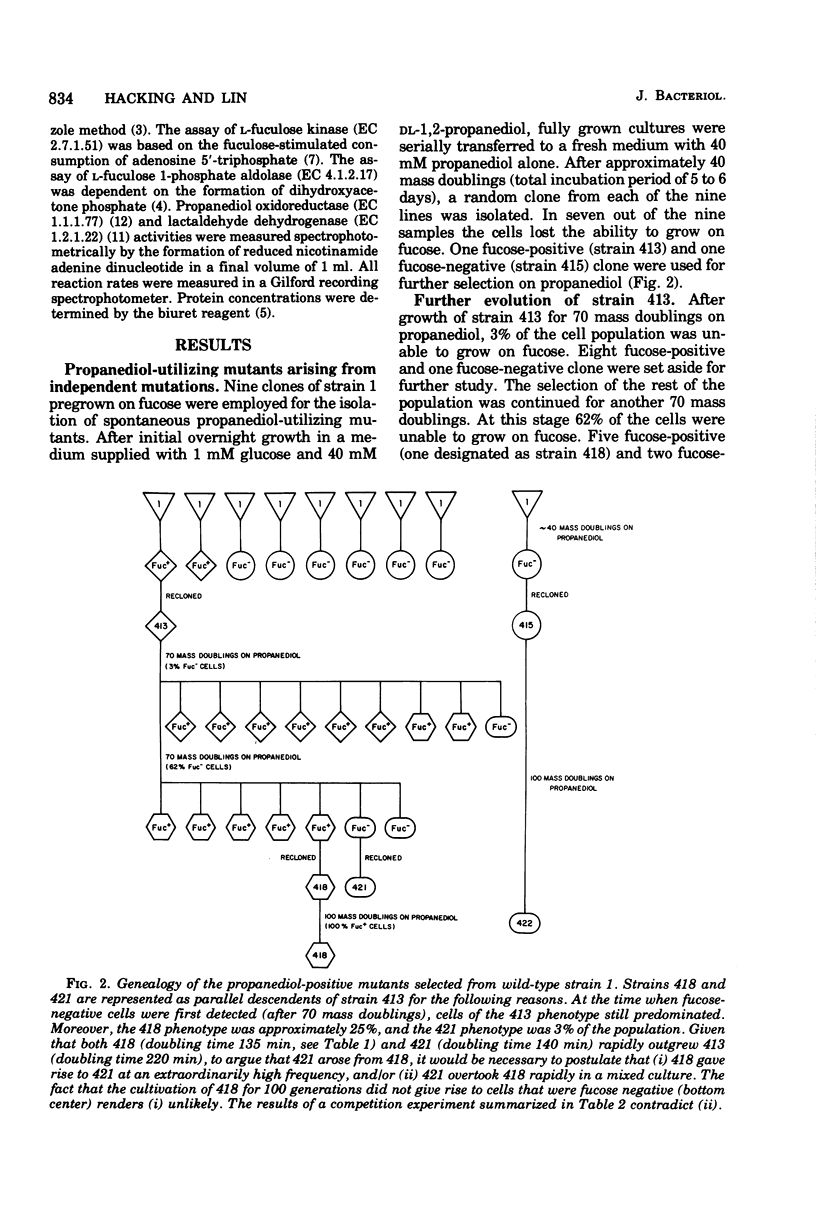
Wild-type strains of Escherichia coli are unable to use L-1,2-propanediol as a carbon and energy source. Strain 3, a mutant selected for the ability to grow on this compound at progressively more rapid rates, synthesizes constitutively a nicotinamide adenine dinucleotide-linked propanediol oxidoreductase. This enzyme is normally synthesized during anaerobic growth on L-fucose when it functions as a lactaldehyde reductase. Propanediol, the end product of this fermentation process, escapes irretrievably into the medium. The propanediol-utilizing mutant can no longer grow on fucose in either the presence or absence of molecular oxygen. In the present study nine independent lines of propanediol-positive mutants were characterized. One mutant, strain 418, attained a propanediol growth rate close to that of strain 3 without loss of the ability to grow on fucose. In all cases examined, however, prolonged selection on propanediol did result in the emergence of fucose-negative mutants. All of these mutants had enzyme patterns similar to that of strain 3; namely, fucose permease, fucose isomerase, and fuculose kinase were noninducible, whereas fuculose 1-phosphate aldolase was constitutive. In strain 418 and in the fucose-positive predecessors of the other mutants, the first four enzymes in the pathway remained inducible, as in the wild-type strain. Improvements in the growth rate on propanediol appeared to reflect principally the increased activity level of the oxidoreductase during the early stages of evolution. According to transductional analysis, the mutations affecting the ability to grow on propanediol and those that affect the expression of the first enzymes in the fucose pathway were very closely linked. The loss of the ability to grow on fucose is thought to be a mechanistic consequence incidental to the remodeling of the regulatory system in favor of the utilization of the novel carbon source.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemohammad M. M., Knowles C. J. Osmotically induced volume and turbidity changes of Escherichia coli due to salts, sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J Gen Microbiol. 1974 May;82(1):125–142. doi: 10.1099/00221287-82-1-125. [DOI] [PubMed] [Google Scholar]
- Cocks G. T., Aguilar T., Lin E. C. Evolution of L-1, 2-propanediol catabolism in Escherichia coli by recruitment of enzymes for L-fucose and L-lactate metabolism. J Bacteriol. 1974 Apr;118(1):83–88. doi: 10.1128/jb.118.1.83-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- HEATH E. C., GHALAMBOR M. A. The metabolism of L-fucose. I. The purification and properties of L-fuculose kinase. J Biol Chem. 1962 Aug;237:2423–2426. [PubMed] [Google Scholar]
- Hacking A. J., Lin E. C. Disruption of the fucose pathway as a consequence of genetic adaptation to propanediol as a carbon source in Escherichia coli. J Bacteriol. 1976 Jun;126(3):1166–1172. doi: 10.1128/jb.126.3.1166-1172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Saint Martin E. J., Mortlock R. P. Natural and altered induction of the L-fucose catabolic enzymes in Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):91–97. doi: 10.1128/jb.127.1.91-97.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T. Purification and properties of lactaldehyde dehydrogenase from Escherichia coli. J Biol Chem. 1969 Oct 10;244(19):5233–5238. [PubMed] [Google Scholar]
- Zagalak B., Frey P. A., Karabatsos G. L., Abeles R. H. The stereochemistry of the conversion of D and L 1,2-propanediols to propionaldehyde. J Biol Chem. 1966 Jul 10;241(13):3028–3035. [PubMed] [Google Scholar]



