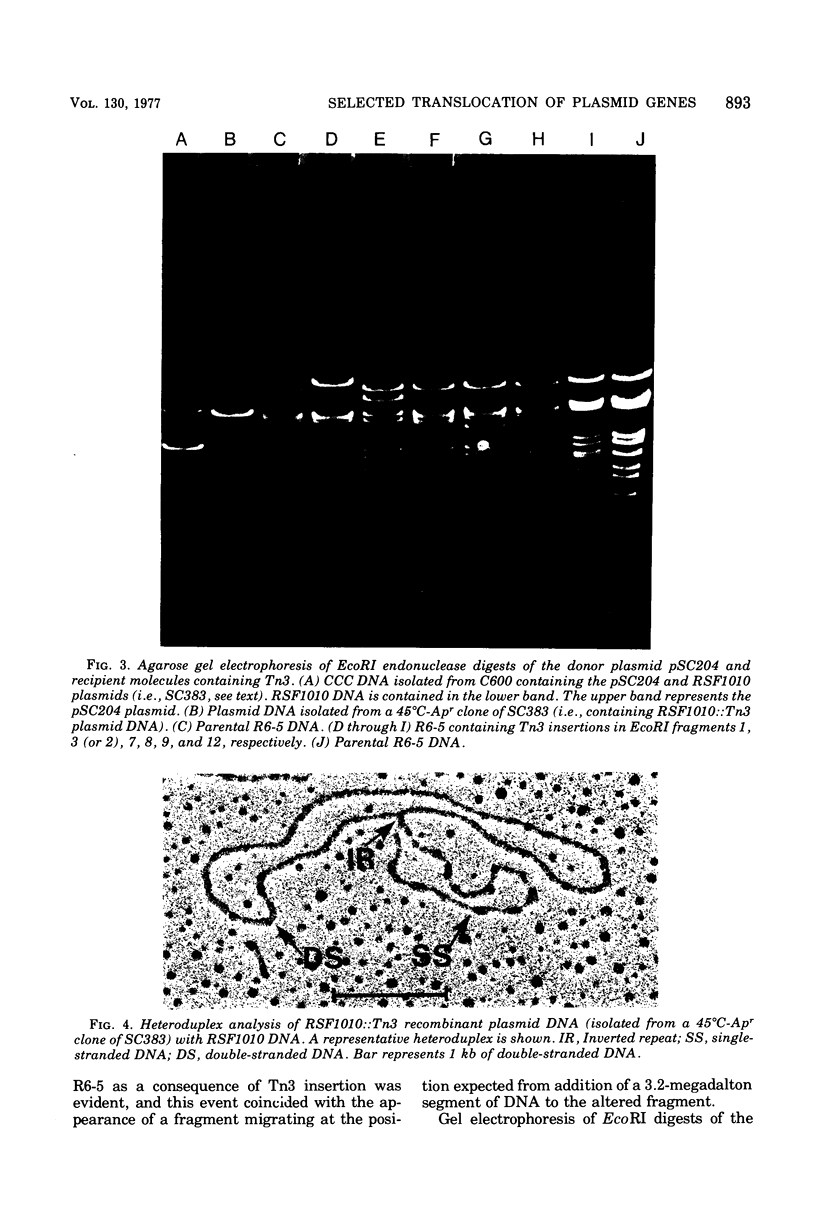
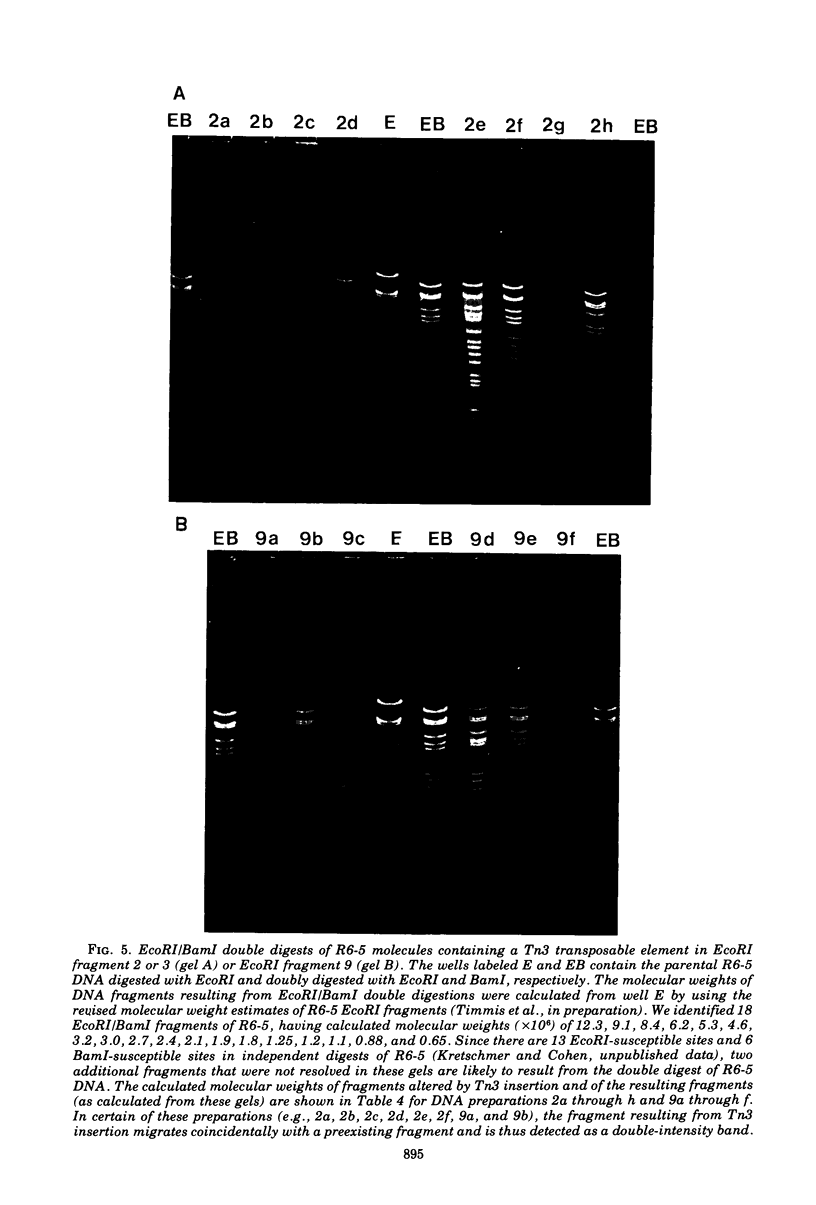
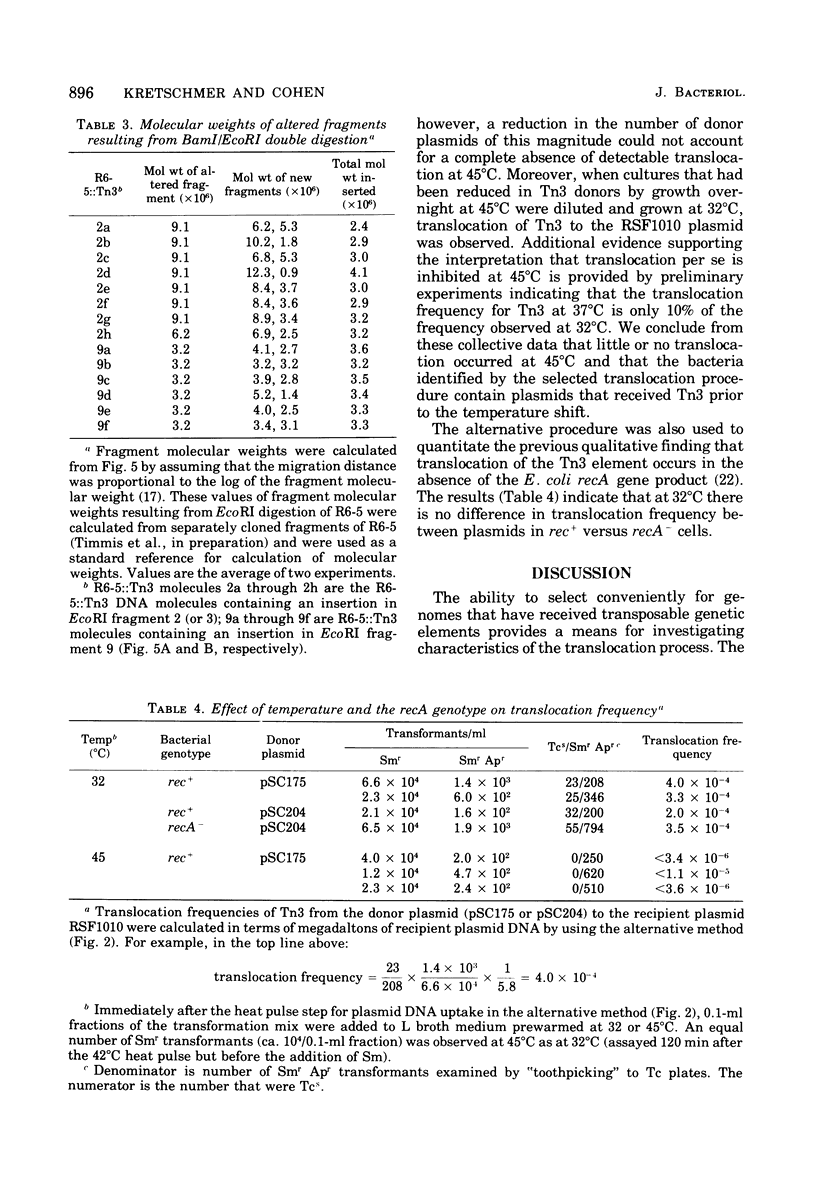
Abstract
A procedure is described that selects for the insertion of transposable antibiotic resistance elements in a variety of recipient replicons. The selected translocation procedure, which employs a plasmid having a temperature-sensitive defect in replication as a donor of transposable genetic elements, was used to investigate certain characteristics of the translocation process. Our results indicate that translocation of the Tn3 element from plasmid to plasmid occurs at a 10(3)- to 10(4)-times-higher frequency than from plasmid to chromosome. In both cases, continued accumulation of Tn3 on recipient genomes is prevented by development of an apparent equilibrium when only a small fraction of molecules in the recipient population contain Tn3. An alternative method for estimation of translocation frequency has shown that the translocation process is temperature sensitive and that its frequency is unaffected by the presence of host recA mutation. Insertions of Tn3 onto the 65 X 10(6)-dalton R6-5 plasmid in Escherichia coli are clustered on EcoRI fragments 3 (8 of 23 insertions) and 9 (7 of 23 insertions), which contain 12 and 5%, respectively, of the R6-5 genome. The occurrence of multiple insertions of Tn3 within EcoRI fragment 9, which contains the IS1 element and a terminus of the Tn4 element, is consistent with earlier evidence indicating that terminal deoxyribonucleic acid sequences of already present transposable elements may provide recognition sequences for subsequent illegitimate recombinational events.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. A method for selective cloning of eukaryotic DNA fragments in Escherichia coli by repeated transformation. Mol Gen Genet. 1974;134(2):133–141. doi: 10.1007/BF00268415. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Kopecko D. J. Structural evolution of bacterial plasmids: role of translocating genetic elements and DNA sequence insertions. Fed Proc. 1976 Jul;35(9):2031–2036. [PubMed] [Google Scholar]
- Cohen S. N. Transposable genetic elements and plasmid evolution. Nature. 1976 Oct 28;263(5580):731–738. doi: 10.1038/263731a0. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Kleid D., Humayun Z., Jeffrey A., Ptashne M. Novel properties of a restriction endonuclease isolated from Haemophilus parahaemolyticus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):293–297. doi: 10.1073/pnas.73.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Brevet J., Cohen S. N. Involvement of multiple translocating DNA segments and recombinational hotspots in the structural evolution of bacterial plasmids. J Mol Biol. 1976 Dec;108(2):333–360. doi: 10.1016/s0022-2836(76)80124-6. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Cohen S. N. Site specific recA--independent recombination between bacterial plasmids: involvement of palindromes at the recombinational loci. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer F. J., Chang A. C., Cohen S. N. Indirect selection of bacterial plasmids lacking identifiable phenotypic properties. J Bacteriol. 1975 Oct;124(1):225–231. doi: 10.1128/jb.124.1.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Lautenberger J. A., Eskin B., Lackey D. Host-controlled restriction and modification enzymes of Escherichia coli B. Fed Proc. 1974 May;33(5):1128–1134. [PubMed] [Google Scholar]
- Reif H. J., Saedler H. IS1 is involved in deletion formation in the gal region of E. coli K12. Mol Gen Genet. 1975;137(1):17–28. doi: 10.1007/BF00332538. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Cohen S. N. Nonchromosomal antibiotic resistance in bacteria. V. Isolation and characterization of R factor mutants exhibiting temperature-sensitive repression of fertility. J Bacteriol. 1972 Jun;110(3):1082–1088. doi: 10.1128/jb.110.3.1082-1088.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]