Abstract
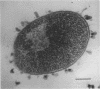
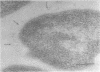
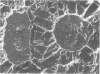
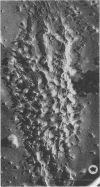
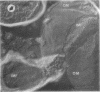
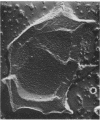
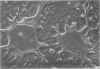
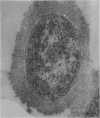
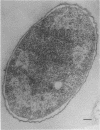
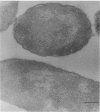
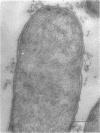
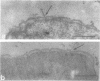
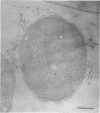
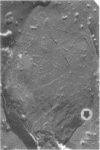
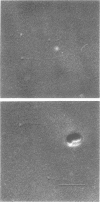
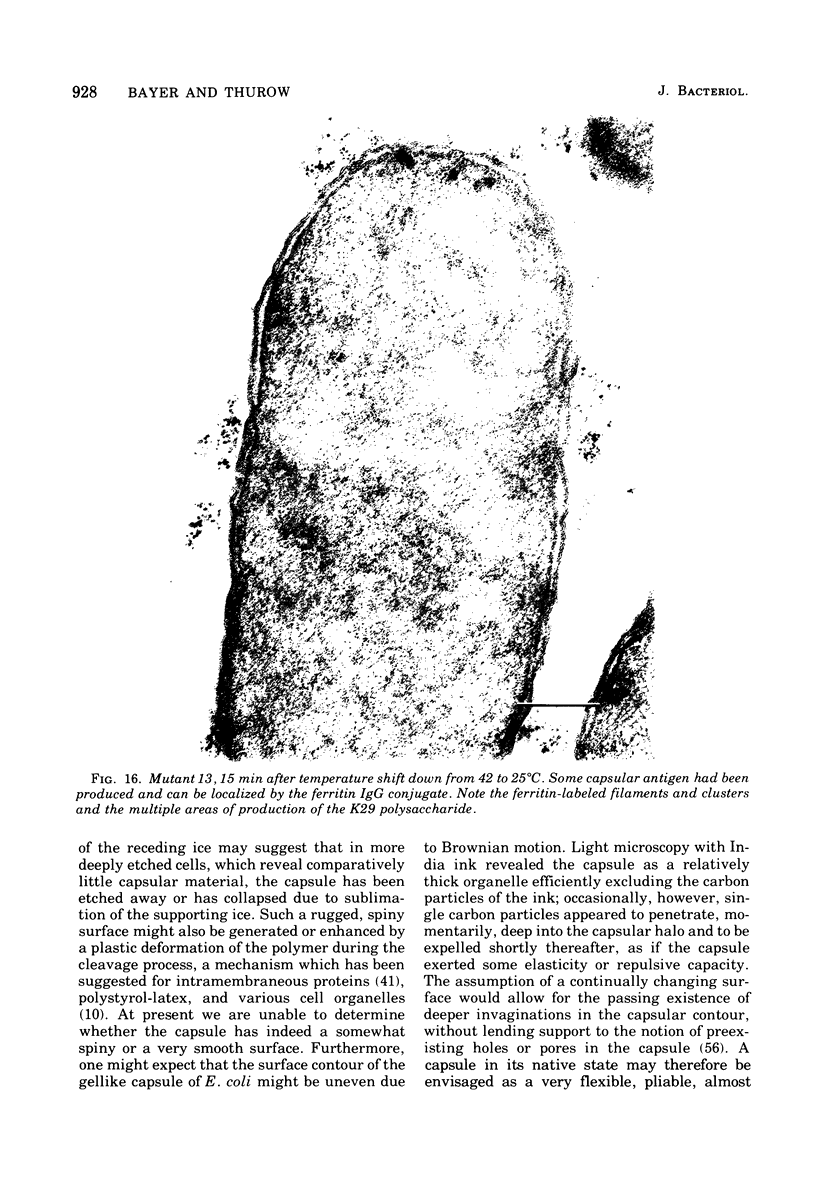
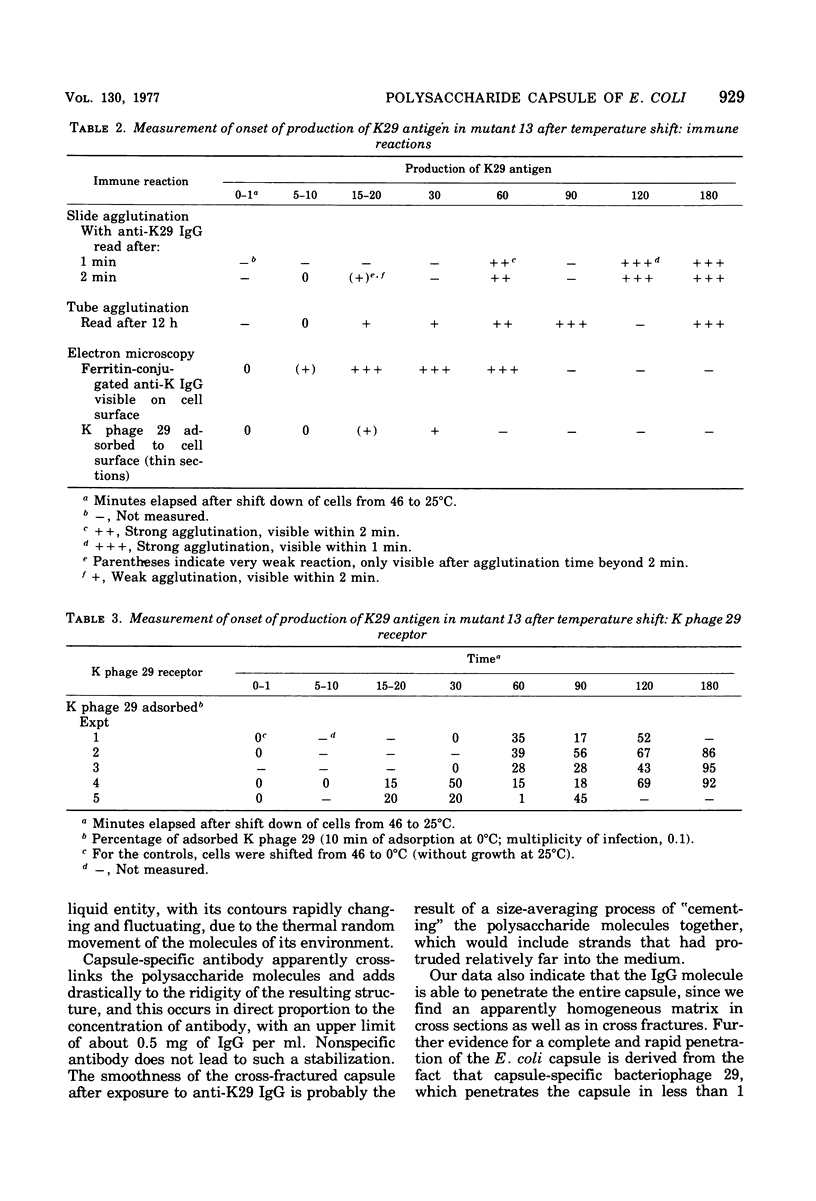
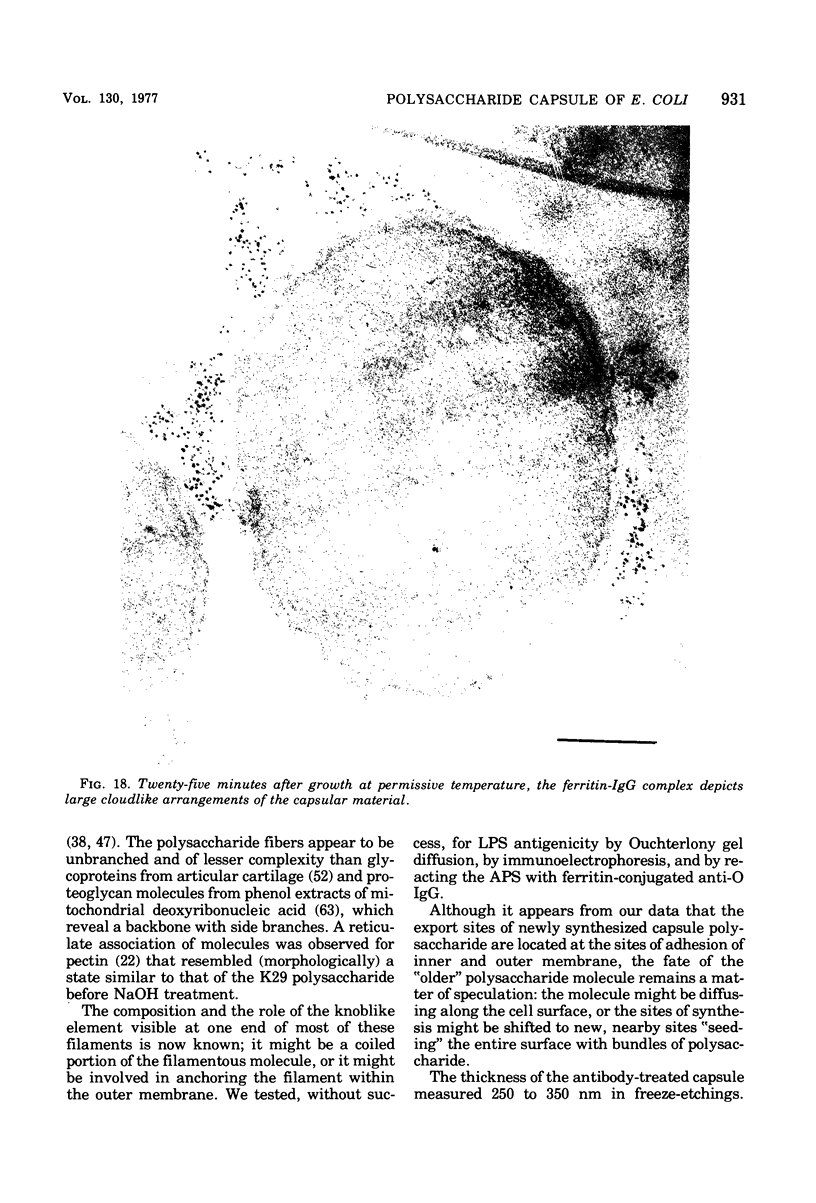
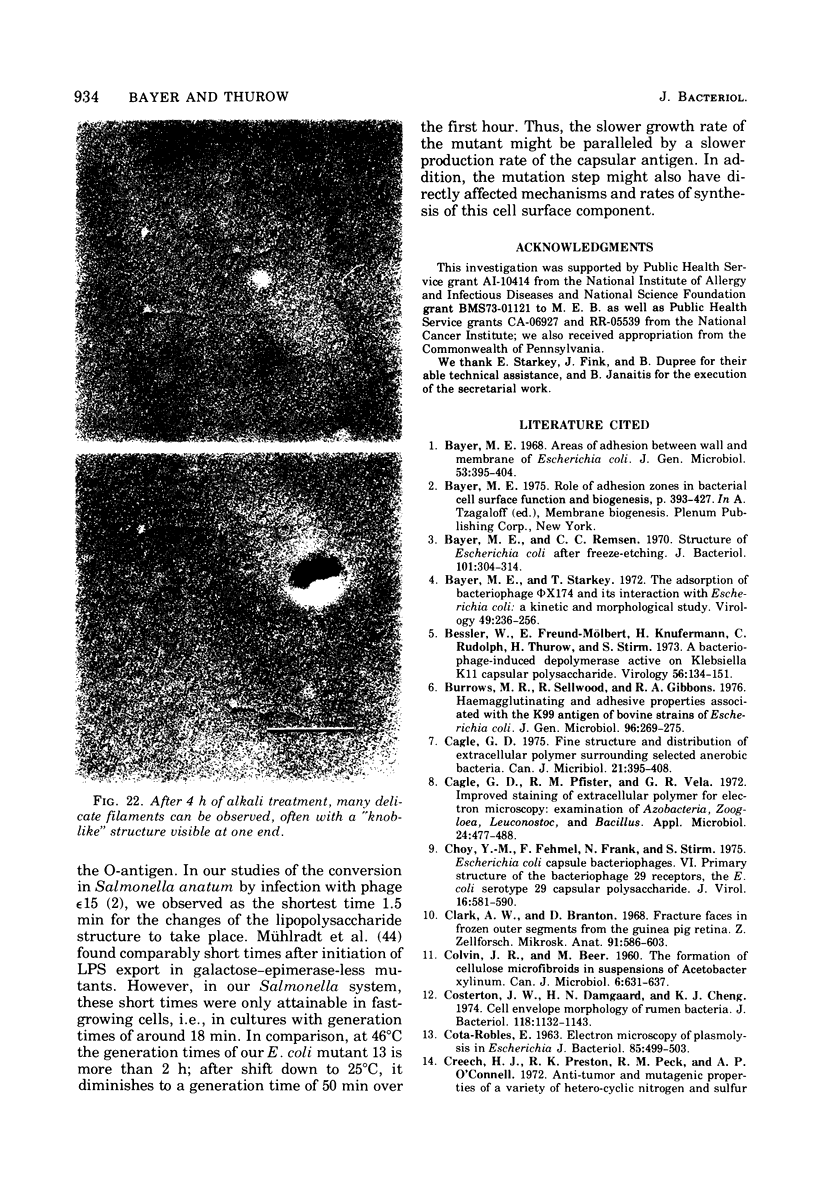
This report describes the structure, size, and shape of the uncollapsed polysaccharide capsule of Escherichia coli strain Bi 161/42 [O9:K29(A):H-], its ultrastructural preservation as well as the filamentous components of the isolated capsular material. In a temperature-sensitive mutant, sites were localized at which capsular polysaccharide is "exported" to the cell surface. The highly hydrated capsule of the wild-type cells was visible in the uncollapsed state after freeze-etching, whereas dehydration in greater than or equal to 50% acetone or alcohol caused the capsule to collapse into thick bundles. This was prevented by pretreatment of the cell with capsule-specific immunoglobulin G; the capsule appeared as a homogeneous layer of 250- to 300-nm thickness. The structural preservation depended on the concentration of the anti-capsular immunoglobulin G. Temperature-sensitive mutants, unable to produce capsular antigen at elevated temperatures, showed, 10 to 15 min after shift down to permissive temperature, polysaccharide strands with K29 specificity appearing at the cell surface at roughly 20 sites per cell; concomitantly, capsule-directed antibody started to agglutinate the bacteria. The sites at which the new antigen emerged were found in random distribution over the entire surface of the organism. Spreading of purified polysaccharide was achieved on air-water interfaces; after subsequent shadow casting with heavy metal, filamentous elements were observed with a smallest class of filaments measuring 250 nm in length and 3 to 6 nm in width. At one end these fibers revealed a knoblike structure of about 10-nm diameter. The slimelike polysaccharides from mutants produced filamentous bundles of greater than 100-microns length, with antigenic and phage-receptor properties indistinguishable from those of the wild-type K29 capsule antigen.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Starkey T. W. The adsorption of bacteriophage phi X174 and its interaction with Escherichia coli; a kinetic and morphological study. Virology. 1972 Jul;49(1):236–256. doi: 10.1016/s0042-6822(72)80026-6. [DOI] [PubMed] [Google Scholar]
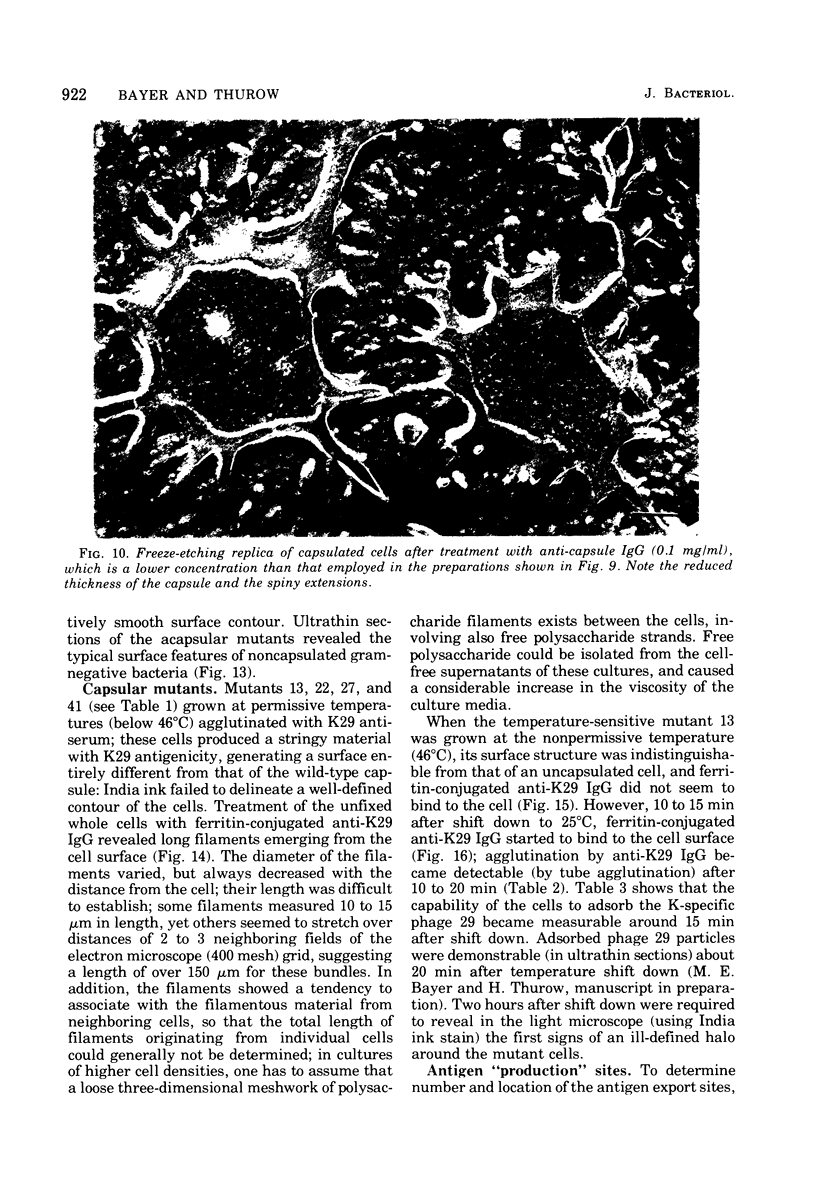
- Bessler W., Freund-Mölbert E., Knüfermann H., Rduolph C., Thurow H., Stirm S. A bacteriophage-induced depolymerase active on Klebsiella K11 capsular polysaccharide. Virology. 1973 Nov;56(1):134–151. doi: 10.1016/0042-6822(73)90293-6. [DOI] [PubMed] [Google Scholar]
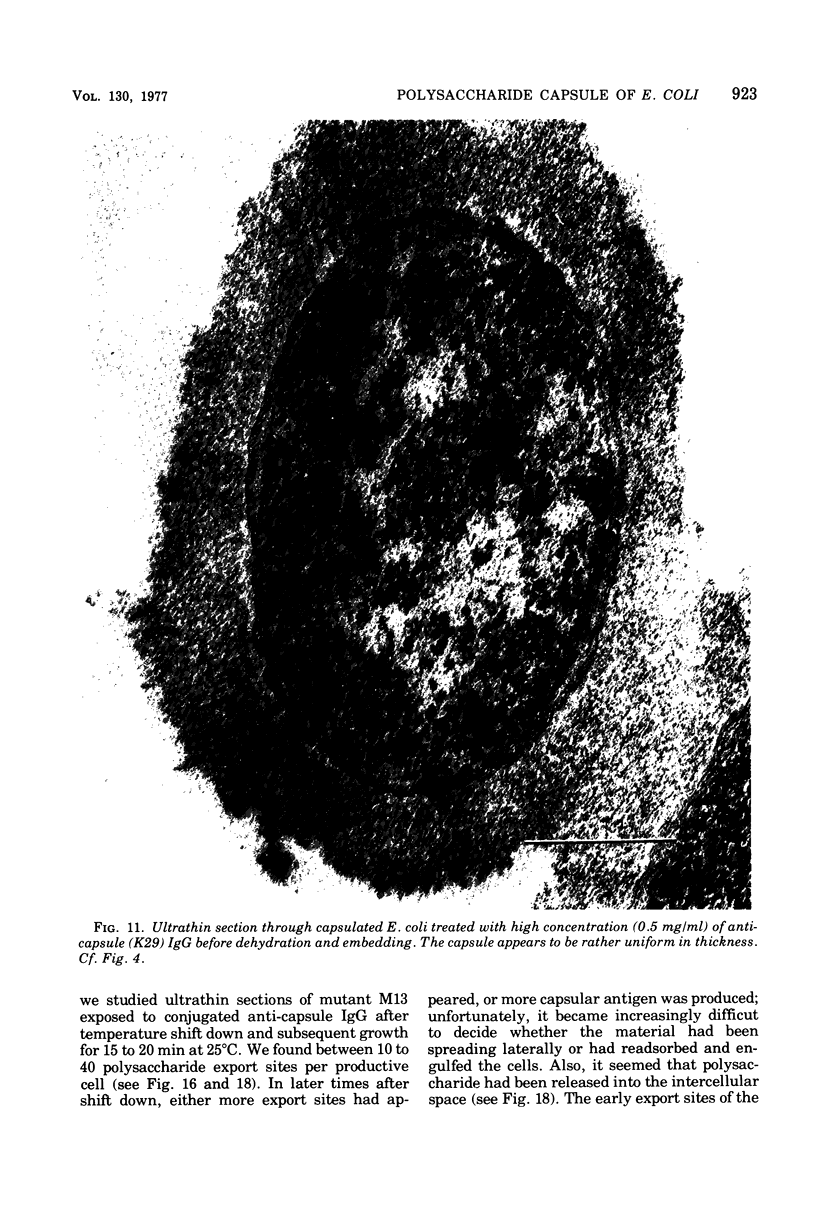
- Burrows M. R., Sellwood R., Gibbons R. A. Haemagglutinating and adhesive properties associated with the K99 antigen of bovine strains of Escherichia coli. J Gen Microbiol. 1976 Oct;96(2):269–275. doi: 10.1099/00221287-96-2-269. [DOI] [PubMed] [Google Scholar]
- COLVIN J. R., BEER M. The formation of cellulose microfibrils in suspensions of Acetobacter xylinum. Can J Microbiol. 1960 Dec;6:631–637. doi: 10.1139/m60-075. [DOI] [PubMed] [Google Scholar]
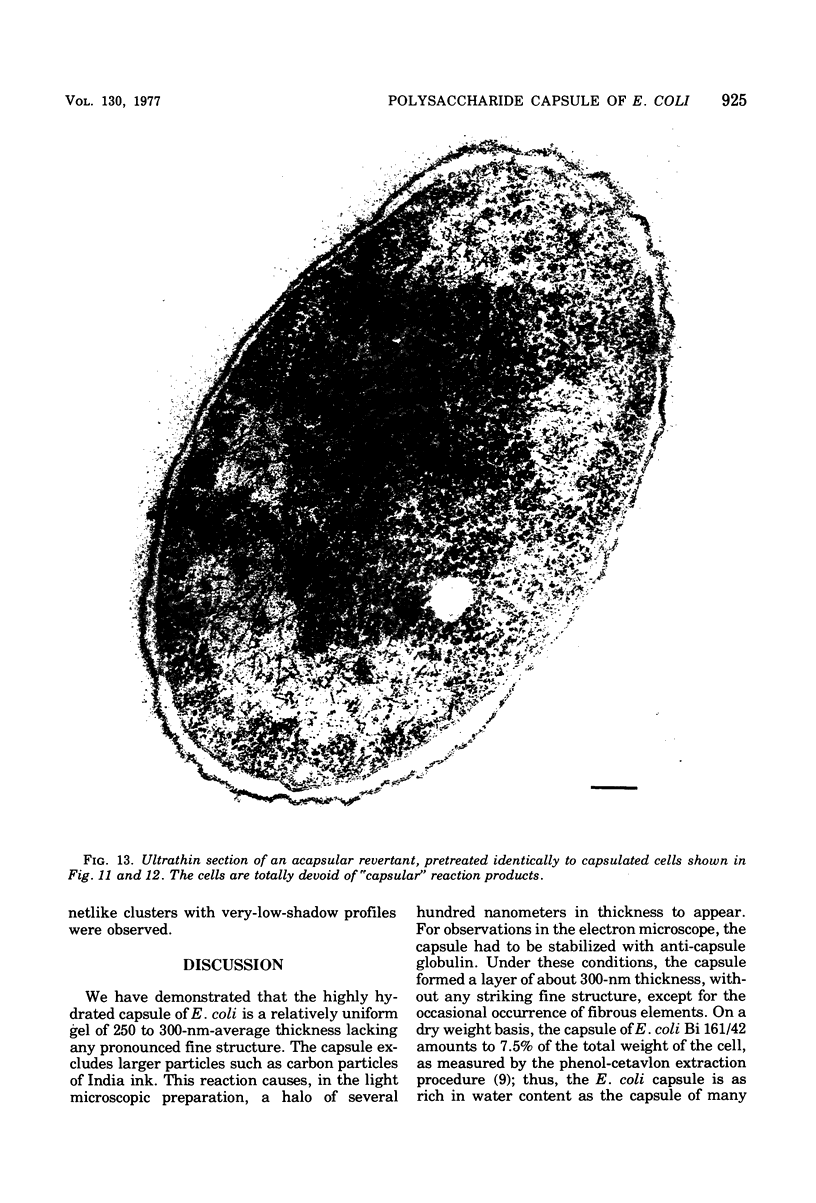
- COTA-ROBLES E. H. ELECTRON MICROSCOPY OF PLASMOLYSIS IN ESCHERICHIA COLI. J Bacteriol. 1963 Mar;85:499–503. doi: 10.1128/jb.85.3.499-503.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D. Fine structure and distribution of extracellular polymer surrounding selected aerobic bacteria. Can J Microbiol. 1975 Mar;21(3):395–408. doi: 10.1139/m75-055. [DOI] [PubMed] [Google Scholar]
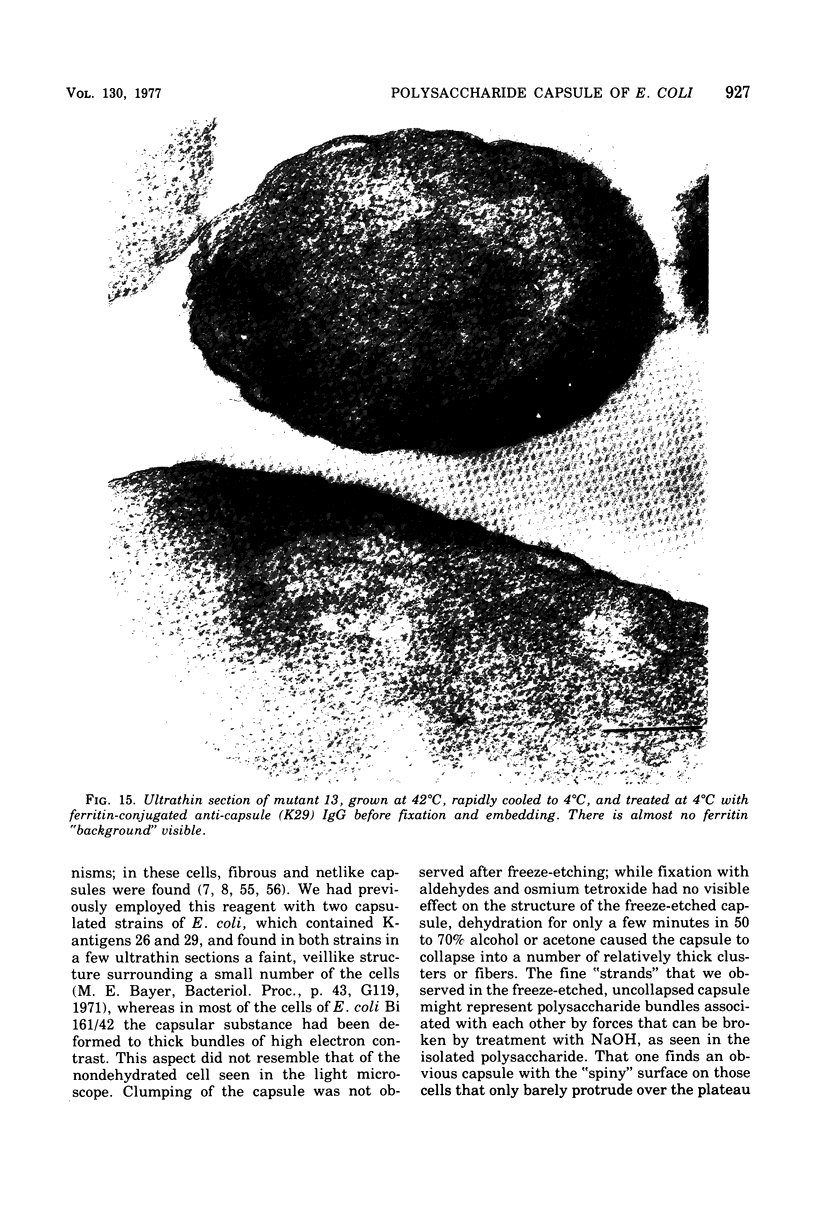
- Cagle G. D., Pfister R. M., Vela G. R. Improved staining of extracellular polymer for electron microscopy: examination of Azotobacter, Zoogloea, Leuconostoc, and Bacillus. Appl Microbiol. 1972 Sep;24(3):477–487. doi: 10.1128/am.24.3.477-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy Y. M., Fehmel F., Frank N., Stirm S. Escherichia coli capsule bacteriophages. IV. Primary structure of the bacteriophage 29 receptor, the E. coli serotype 29 capsular polysaccharide. J Virol. 1975 Sep;16(3):581–590. doi: 10.1128/jvi.16.3.581-590.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Branton D. Fracture faces in frozen outer segments from the guinea pig retina. Z Zellforsch Mikrosk Anat. 1968;91(4):586–603. doi: 10.1007/BF00455276. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech H. J., Preston R. K., Peck R. M., O'Connell A. P. Antitumor and mutagenic properties of a variety of heterocyclic nitrogen and sulfur mustards. J Med Chem. 1972 Jul;15(7):739–746. doi: 10.1021/jm00277a011. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Fehmel F., Feige U., Niemann H., Stirm S. Escherichia coli capsule bacteriophages. VII. Bacteriophage 29-host capsular polysaccharide interactions. J Virol. 1975 Sep;16(3):591–601. doi: 10.1128/jvi.16.3.591-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Grant W. D., Sutherland I. W., Wilkinson J. F. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J Bacteriol. 1969 Dec;100(3):1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke D. E., Northcote D. H. Molecular visualization of pectin and DNA by ruthenium red. Biopolymers. 1975 Jan;14(1):1–17. doi: 10.1002/bip.1975.360140102. [DOI] [PubMed] [Google Scholar]
- Hungerer D., Jann K., Jann B., Orskov F., Orskov I. Immunochemistry of K antigens of Escherichia coli. 4. The K antigen of E. coli O 9:K30:H12. Eur J Biochem. 1967 Jul;2(1):115–126. doi: 10.1111/j.1432-1033.1967.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Jann K., Jann B., Orskov F., Orskov I., Westphal O. Immunchemische Untersuchungen an K-Antigenen von Escherichia Coli. II. Das K-Antigen von E. coli 08:K42(A):H-. Biochem Z. 1965 Jun 3;342(1):1–22. [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser G. E., Starzyk M. J. Ultrastructure and cell division of an oral bacterium resembling Alysiella filiformis. Can J Microbiol. 1973 Mar;19(3):325–327. doi: 10.1139/m73-054. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Molecular interactions between the extracellular polysaccharides of Streptococcus mutans. Arch Oral Biol. 1972 Dec;17(12):1659–1670. doi: 10.1016/0003-9969(72)90228-2. [DOI] [PubMed] [Google Scholar]
- Klein P. A., Adams W. R. Location of ferritin-labeled concanavalin A binding to influenza virus and tumor cell surfaces. J Virol. 1972 Oct;10(4):844–854. doi: 10.1128/jvi.10.4.844-854.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABAW L. W., MOSLEY V. M. Demonstration of striated fibers in the capsule of the Lisbonne strain of lysogenic Escherichia coli. J Bacteriol. 1954 May;67(5):576–584. doi: 10.1128/jb.67.5.576-584.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Ba-Nhan, Jann B., Jann K. Immunochemistry of K antigens of Escherichia coli. The K29 antigen of E. coli 09:K29(A):H-. Eur J Biochem. 1971 Jul 29;21(2):226–234. doi: 10.1111/j.1432-1033.1971.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Meyer H. W., Winkelmann H. Uber die Anordnung der Membranproteine nach Untersuchungen mit der Gefrierätzung an isolierten Erythozytenmembranen. Protoplasma. 1972;75(3):255–284. doi: 10.1007/BF01279819. [DOI] [PubMed] [Google Scholar]
- Moorhouse R., Winter W. T., Arnott S., Bayer M. E. Conformation and molecular organization in fibers of the capsular polysaccharide from Escherichia coli M41 mutant. J Mol Biol. 1977 Jan 25;109(3):373–391. doi: 10.1016/s0022-2836(77)80018-1. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Outer membrane of salmonella. Sites of export of newly synthesised lipopolysaccharide on the bacterial surface. Eur J Biochem. 1973 Jun 15;35(3):471–481. doi: 10.1111/j.1432-1033.1973.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., JANN B., JANN K. ACIDIC POLYSACCHARIDE ANTIGENS OF A NEW TYPE FROM E. COLI CAPSULES. Nature. 1963 Oct 12;200:144–146. doi: 10.1038/200144a0. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Schmidt G. Basalstrukturen von verschiedenen Lipopolysacchariden verschiedener Enterobakteriaceen. Genetische und serologische Untersuchungen an R-Mutanten. Zentralbl Bakteriol Orig A. 1972 May;220(1):472–476. [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. Ultrastructure of the capsule of Klebsiella pneumoniae and slime of Enterobacter aerogenes revealed by freeze etching. Arch Mikrobiol. 1973 Nov 19;93(4):277–286. doi: 10.1007/BF00427925. [DOI] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E. Bacteriophage particles with endo-glycosidase activity. J Virol. 1971 Sep;8(3):343–346. doi: 10.1128/jvi.8.3.343-346.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E., Thurow H. Isolation of spike-formed particles from bacteriophage lysates. Virology. 1971 Jul;45(1):303–308. doi: 10.1016/0042-6822(71)90138-3. [DOI] [PubMed] [Google Scholar]
- Stirm S., Freund-Mölbert E. Escherichia coli capsule bacteriophages. II. Morphology. J Virol. 1971 Sep;8(3):330–342. doi: 10.1128/jvi.8.3.330-342.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Birch-Andersen A. Episome-carried surface antigen K88 of Escherichia coli. 3. Morphology. J Bacteriol. 1967 Feb;93(2):740–748. doi: 10.1128/jb.93.2.740-748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. Physical and chemical changes of Vi-polysaccharide due to Vi-phage II action. Acta Biochim Pol. 1966;13(1):97–106. [PubMed] [Google Scholar]
- Thurow H., Niemann H., Rudolph C., Stirm S. Host capsule depolymerase activity of bacteriophage particles active on Klebsiella K20 and K24 strains. Virology. 1974 Mar;58(1):306–309. doi: 10.1016/0042-6822(74)90166-4. [DOI] [PubMed] [Google Scholar]
- WILKINSON J. F., DUGUID J. P., EDMUNDS P. N. The distribution of polysaccharide production in Aerobacter and Escherichia strains and its relation to antigenic character; with a note on the influence of potassium deficiency upon production of polysaccharide by Aerobacter aerogenes. J Gen Microbiol. 1954 Aug;11(1):59–72. doi: 10.1099/00221287-11-1-59. [DOI] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P., Weber R. A structure resembling proteinpolysaccharide complexes in preparations of mitochondrial DNA from mouse liver. Experientia. 1971 Oct 15;27(10):1171–1173. doi: 10.1007/BF02286907. [DOI] [PubMed] [Google Scholar]