Abstract
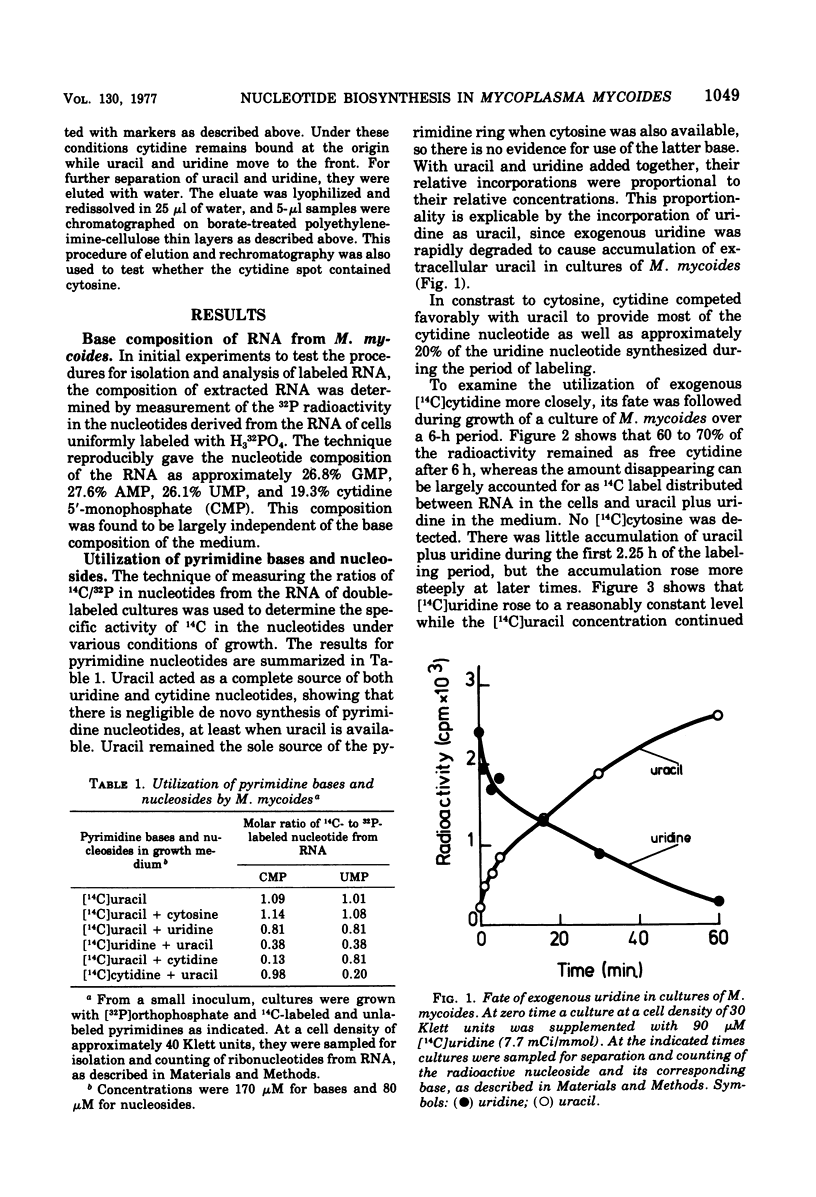
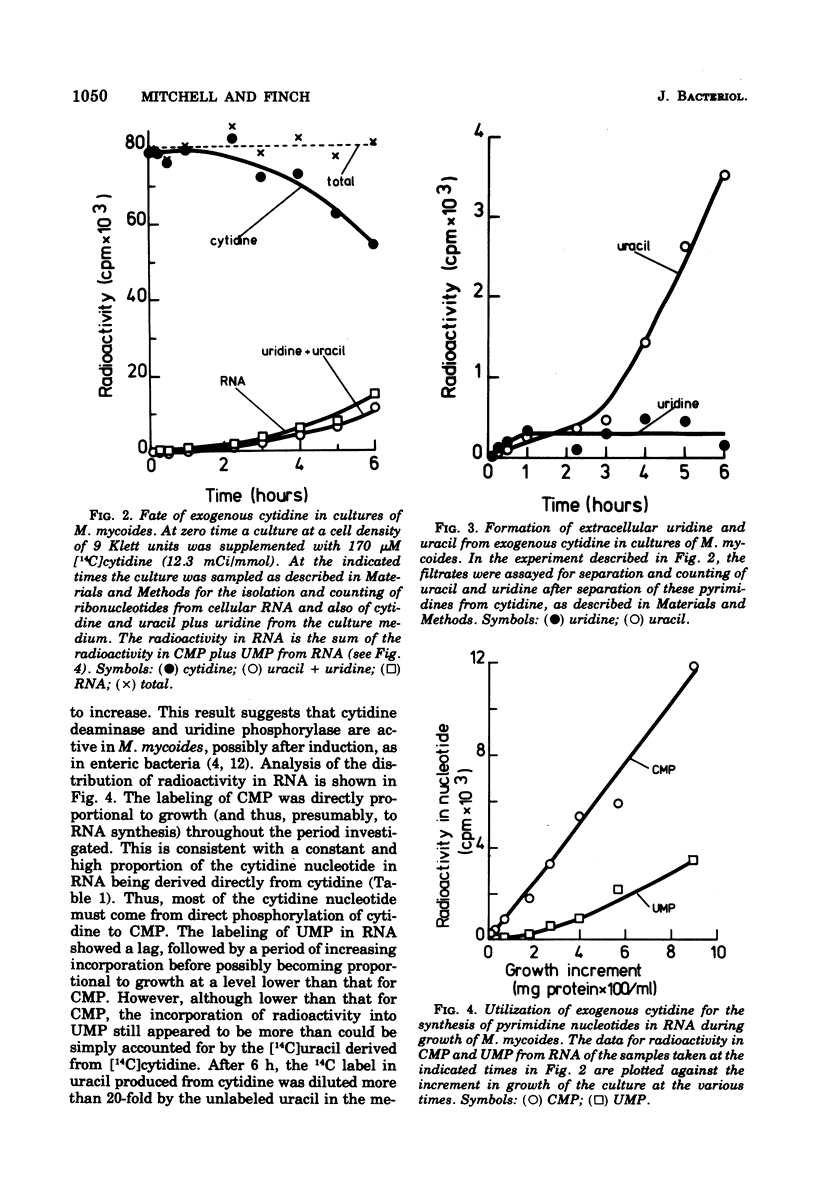
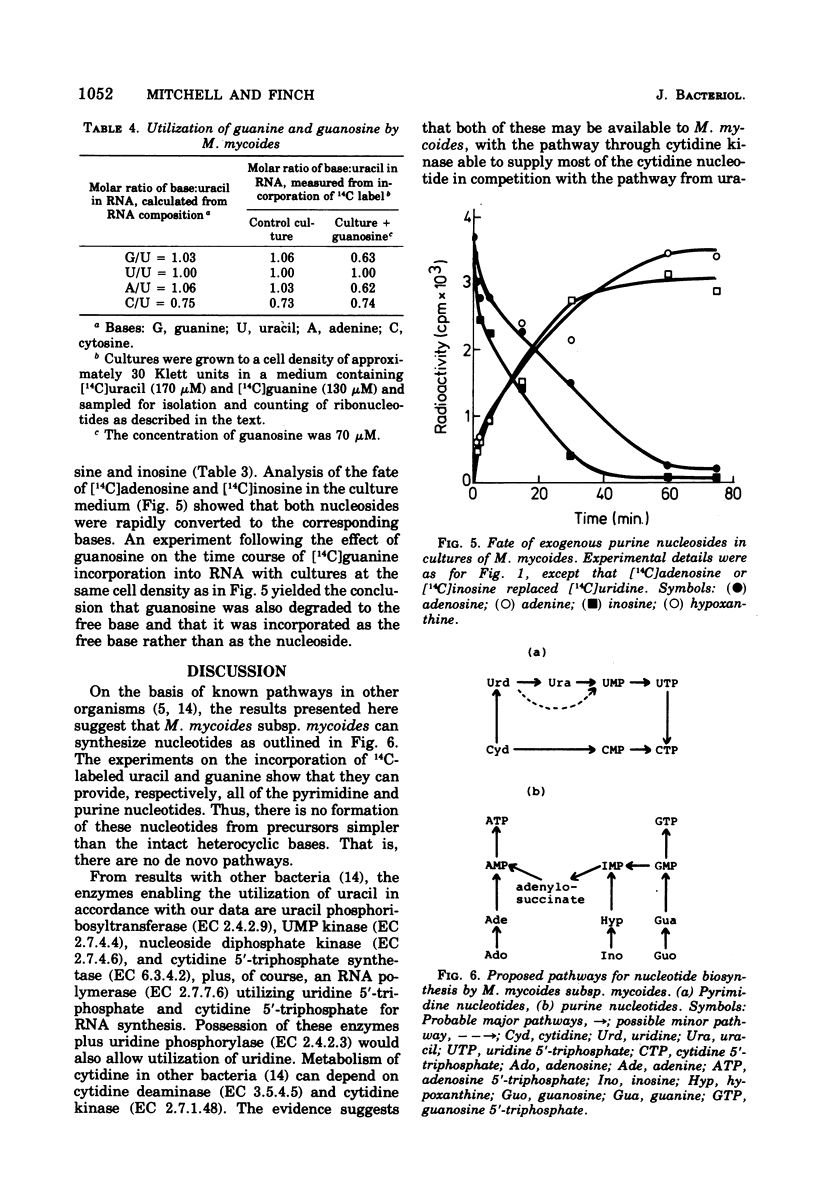
By measuring the specific activity of nucleotides isolated from ribonucleic acid after the incorporation of 14C-labeled precursors under various conditions of growth, we have defined the major pathways of ribonucleotide synthesis in Mycoplasma mycoides subsp. mycoides. M. mycoides did not possess pathways for the de novo synthesis of nucleotides but was capable of interconversion of nucleotides. Thus, uracil provided the requirement for both pyrimidine ribonucleotides. Thymine is also required, suggesting that the methylation step is unavailable. No use was made of cytosine. Uridine was rapidly degraded to uracil. Cytidine competed effectively with uracil to provide most of the cytidine nucleotide and also provided an appreciable proportion of uridine nucleotide. In keeping with these results, there was a slow deamination of cytidine to uridine with further degradation to uracil in cultures of M. mycoides. Guanine was capable of meeting the full requirement of the organism for purine nucleotide, presumably by conversion of guanosine 5′-monophosphate to adenosine 5′-monophosphate via the intermediate inosine 5′-monophosphate. When available with guanine, adenine effectively gave a complete provision of adenine nucleotide, whereas hypoxanthine gave a partial provision. Neither adenine nor hypoxanthine was able to act as a precursor for the synthesis of guanine nucleotide. Exogenous guanosine, inosine, and adenosine underwent rapid cleavage to the corresponding bases and so show a pattern of utilization similar to that of the latter.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L., Neuhard J., Thomassen E. Metabolism of pyrimidines and pyrimidine nucleosides by Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):219–228. doi: 10.1128/jb.110.1.219-228.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5'-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969 Dec;11(2):249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A., Schwartz M., Nygaard P. Induction of enzymes involed in the catabolism of deoxyribonucleosides and ribonucleosides in Escherichia coli K 12. Eur J Biochem. 1971 Apr 30;19(4):533–538. doi: 10.1111/j.1432-1033.1971.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. III. The involvement of purine phosphoribosyltransferases in the uptake of adenine and other nucleic acid precursors by intact resting cells. J Biol Chem. 1971 Sep 10;246(17):5312–5320. [PubMed] [Google Scholar]
- Hochstadt-Ozer J. The regulation of purine utilization in bacteria. IV. Roles of membrane-localized and pericytoplasmic enzymes in the mechanism of purine nucleoside transport across isolated Escherichia coli membranes. J Biol Chem. 1972 Apr 25;247(8):2419–2426. [PubMed] [Google Scholar]
- Hoffmeyer J., Neuhard J. Metabolism of exogenous purine bases and nucleosides by Salmonella typhimurium. J Bacteriol. 1971 Apr;106(1):14–24. doi: 10.1128/jb.106.1.14-24.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A., Nygaard P., Hammer-Jespersen K., Fiil N. Mutants constitutive for nucleoside-catabolizing enzymes in Escherichia coli K12. Isolation, charactrization and mapping. Eur J Biochem. 1972 May 23;27(2):208–215. doi: 10.1111/j.1432-1033.1972.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968 Jun;95(6):2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan D. C., Hochstadt J. Group translocation of the ribose moiety of inosine by vesicles of plasma membrane from T(3 cells transformed by Simian virus 40. J Biol Chem. 1976 Jan 25;251(2):344–354. [PubMed] [Google Scholar]
- RODWELL A. W. Nutrition and metabolism of Mycoplasma mycoides var. mycoides. Ann N Y Acad Sci. 1960 Jan 15;79:499–507. doi: 10.1111/j.1749-6632.1960.tb42716.x. [DOI] [PubMed] [Google Scholar]
- Rader R. L., Hochstadt J. Regulation of purine utilization in bacteria. VII. Involvement of membrane-associated nucleoside phosphorylase in the uptake and the base-mediated loss of the ribose moiety of nucleosides by Salmonella typhimurium membrane vesicles. J Bacteriol. 1976 Oct;128(1):290–301. doi: 10.1128/jb.128.1.290-301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Rodwell A. W. The supply of cholesterol and fatty acids for the growth of mycoplasmas. J Gen Microbiol. 1969 Sep;58(1):29–37. doi: 10.1099/00221287-58-1-29. [DOI] [PubMed] [Google Scholar]
- Schrecker A., Jacobsen D. W., Kirchner J. Separation of ribonucleosides from deoxyribonucleosides and arabinonucleosides by thin-layer chromatography. Anal Biochem. 1968 Dec;26(3):474–477. doi: 10.1016/0003-2697(68)90217-0. [DOI] [PubMed] [Google Scholar]
- Sin I. L., Finch L. R. Adenine phosphoribosyltransferase in Mycoplasma mycoides and Escherichia coli. J Bacteriol. 1972 Oct;112(1):439–444. doi: 10.1128/jb.112.1.439-444.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



