Abstract
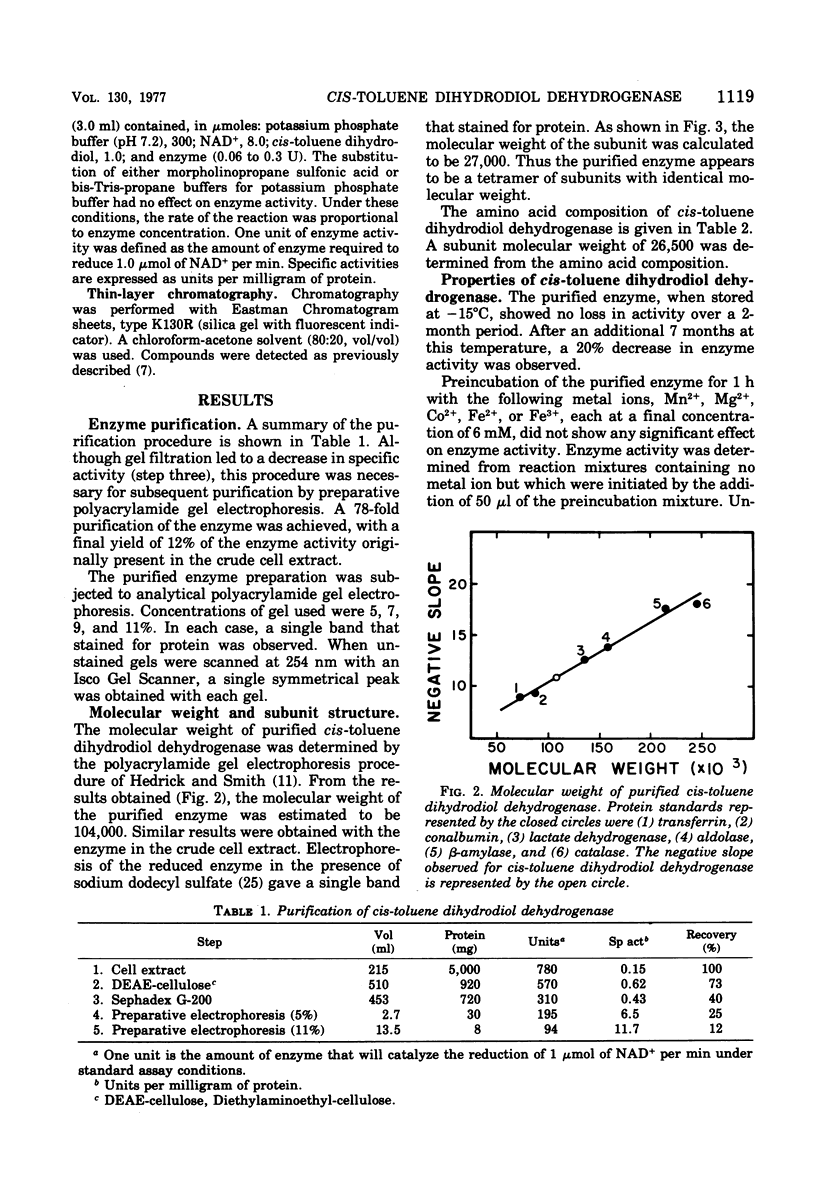
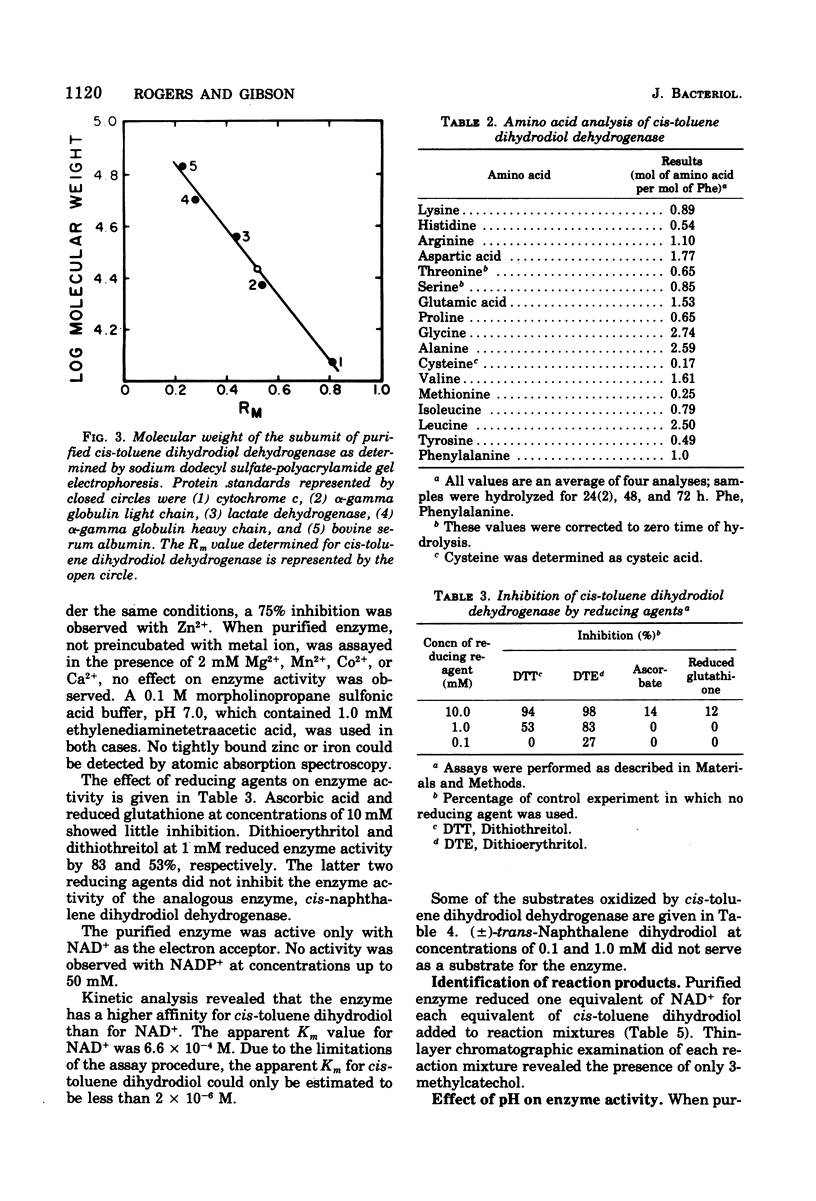
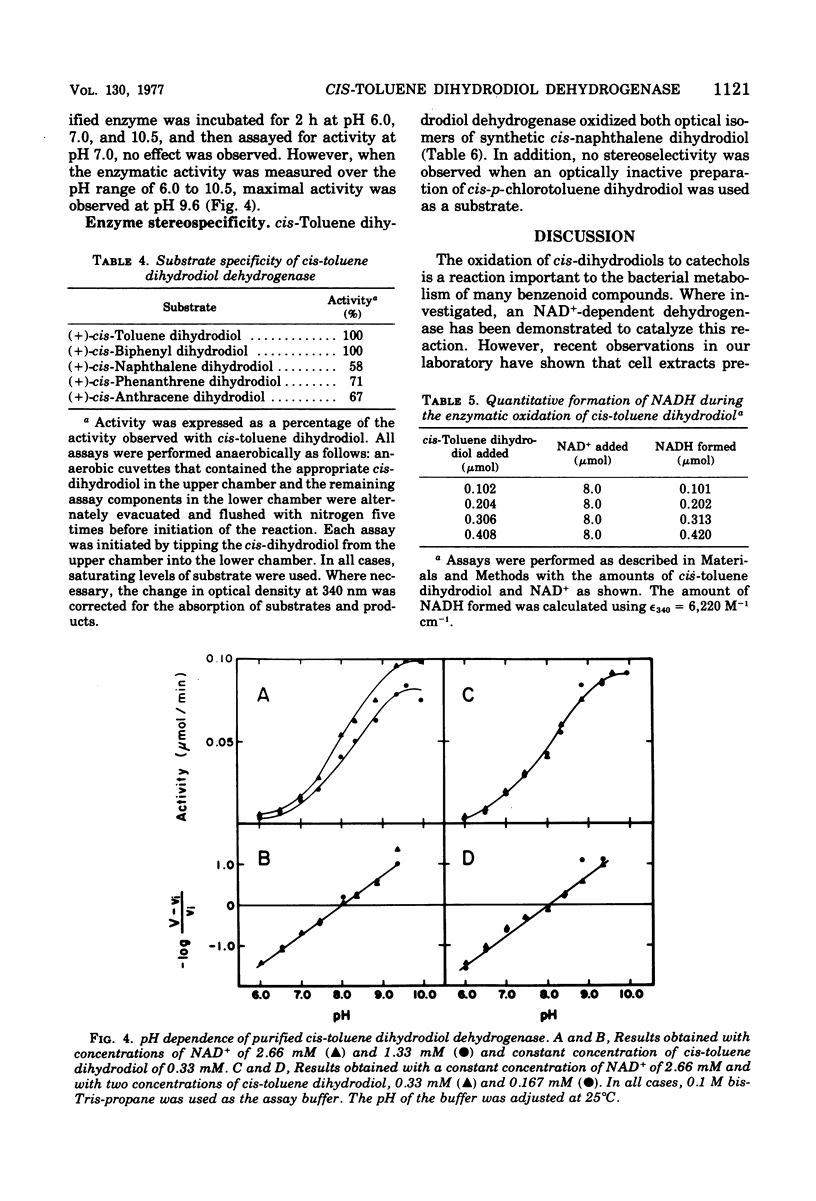
The purification of (+)-cis-1(S),2(R)-dihydroxy-3-methylcyclohexa-3,5-diene dehydrogenase from cells of Pseudomonas putida grown with toluene as the sole source of carbon and energy is reported. The molecular weight of the enzyme is 104,000 at pH 9.7. The enzyme is composed of four apparently identical subunits with molecular weights of 27,000. The enzyme is specific for nicotinamide adenine dinucleotide and oxidizes a number of cis-dihydrodiols. Both enantiomers of a racemic mixture of cis-1,2-dihydroxyl-1,2-dihydronaphthalene dihydrodiol are oxidized by the enzyme. No enzymatic activity is observed with trans-1,2-dihydroxyl-1,2-dihydronaphthalene dihydrodiol.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTY R. A. Enzyme kinetics. Adv Enzymol Relat Subj Biochem. 1956;17:1–64. doi: 10.1002/9780470122624.ch1. [DOI] [PubMed] [Google Scholar]
- AYENGAR P. K., HAYAISHI O., NAKAJIMA M., TOMIDA I. Enzymic aromatization of 3,5-cyclohexadiene-1,2-diol. Biochim Biophys Acta. 1959 May;33(1):111–119. doi: 10.1016/0006-3002(59)90504-9. [DOI] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. The metabolism of benzene by bacteria. Purification and some properties of the enzyme cis-1,2-dihydroxycyclohexa-3,5-diene (nicotinamide adenine dinucleotide) oxidoreductase (cis-benzene glycol dehydrogenase). Biochem J. 1973 Dec;136(4):927–934. doi: 10.1042/bj1360927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S. Catabolism of aromatic compounds by micro-organisms. Adv Microb Physiol. 1971;6(0):1–46. doi: 10.1016/s0065-2911(08)60066-1. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Gschwendt B., Yeh W. K., Kobal V. M. Initial reactions in the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry. 1973 Apr 10;12(8):1520–1528. doi: 10.1021/bi00732a008. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Schuld C. L., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry. 1968 Nov;7(11):3795–3802. doi: 10.1021/bi00851a003. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Haschke R. H., Campbell L. L. Purification and properties of a hydrogenase from Desulfovibrio vulgaris. J Bacteriol. 1971 Jan;105(1):249–258. doi: 10.1128/jb.105.1.249-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Yeh H. J., Jerina D. M., Patel T. R., Davey J. F., Gibson D. T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975 Feb 11;14(3):575–584. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Selander H., Yagi H., Wells M. C., Davey J. F., Mahadevan V., Gibson D. T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976 Sep 15;98(19):5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- Knackmuss H. J., Beckmann W., Otting W. Microbiological synthesis of (+)-cis-1,2-dihydroxy-1,2-dihydronaphthalene-2-carboxylic acid. Angew Chem Int Ed Engl. 1976 Sep;15(9):549–549. doi: 10.1002/anie.197605491. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oesch F., Daly J. Conversion of naphthalene to trans-naphthalene dihydrodiol: evidence for the presence of a coupled aryl monooxygenase-epoxide hydrase system in hepatic microsomes. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1713–1720. doi: 10.1016/0006-291x(72)90807-8. [DOI] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Bacterial cis-dihydrodiol dehydrogenases: comparison of physicochemical and immunological protperties. J Bacteriol. 1976 Dec;128(3):842–850. doi: 10.1128/jb.128.3.842-850.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of aromatic compounds in bacteria. Purification and properties of the catechol-forming enzyme, 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (NAD + ) oxidoreductase (decarboxylating). J Biol Chem. 1972 Aug 25;247(16):4960–4965. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- TANIUCHI H., HAYAISHI O. Studies on the metabolism of kynurenic acid. III. Enzymatic formation of 7,8-dihydroxykynurenic acid from kynurenic acid. J Biol Chem. 1963 Jan;238:283–293. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Ziffer H., Jerina D. M., Gibson D. T., Kobal V. M. Absolute stereochemistry of the (+)-cis-1,2-dihydroxy-3-methylcyclohexa-3,5-diene produced from toluene by Pseudomonas putida. J Am Chem Soc. 1973 Jun 13;95(12):4048–4049. doi: 10.1021/ja00793a036. [DOI] [PubMed] [Google Scholar]



