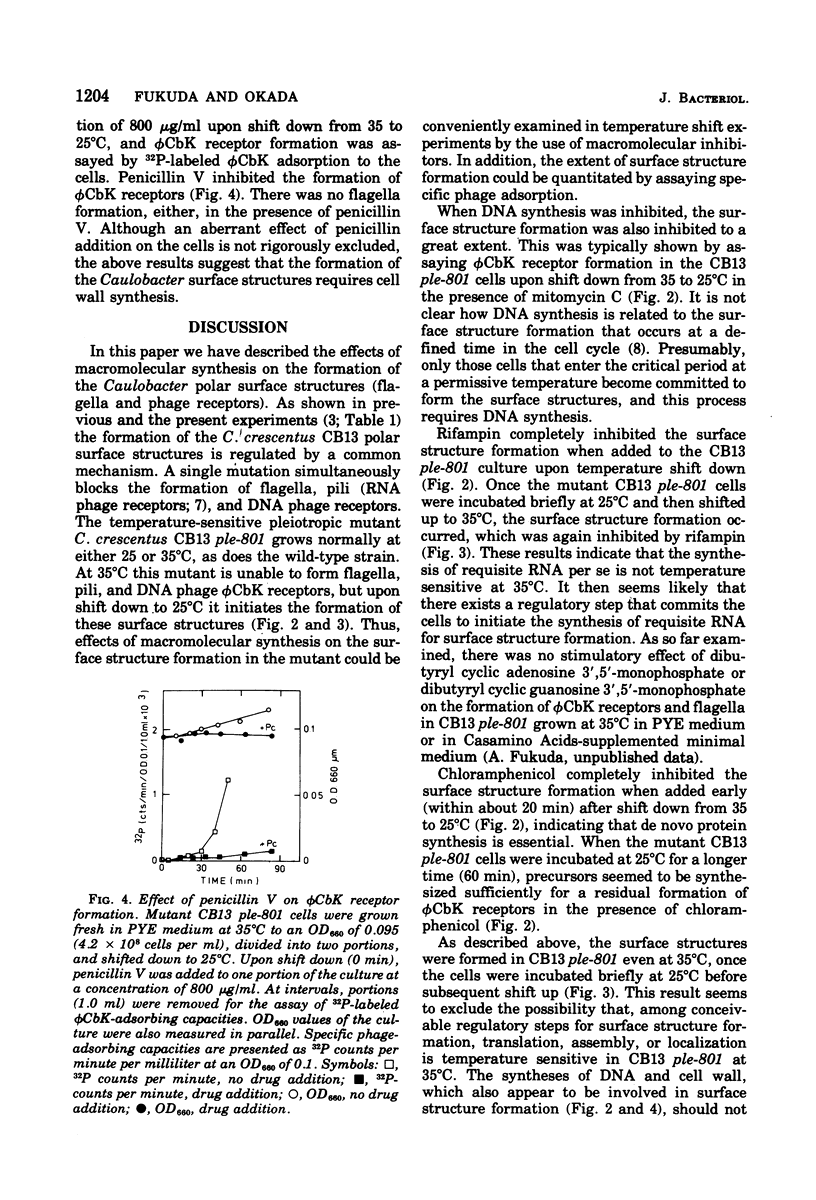
Abstract
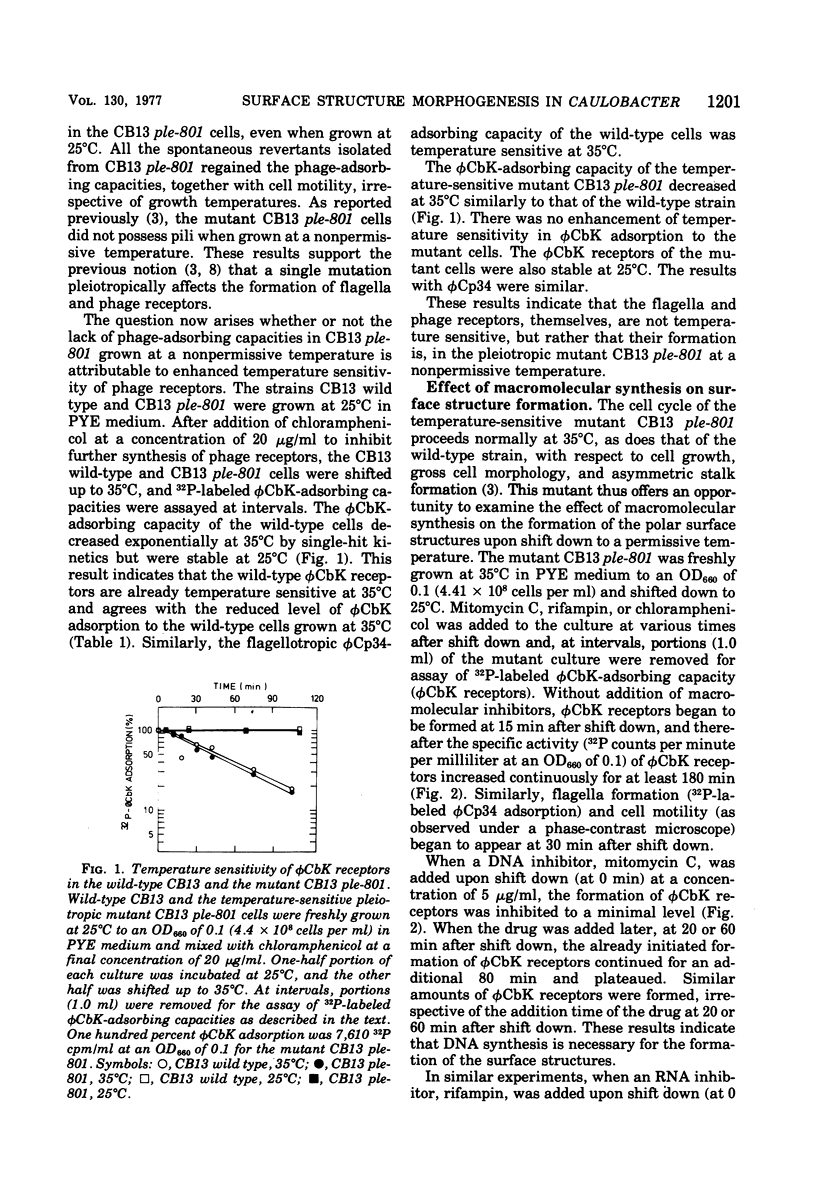
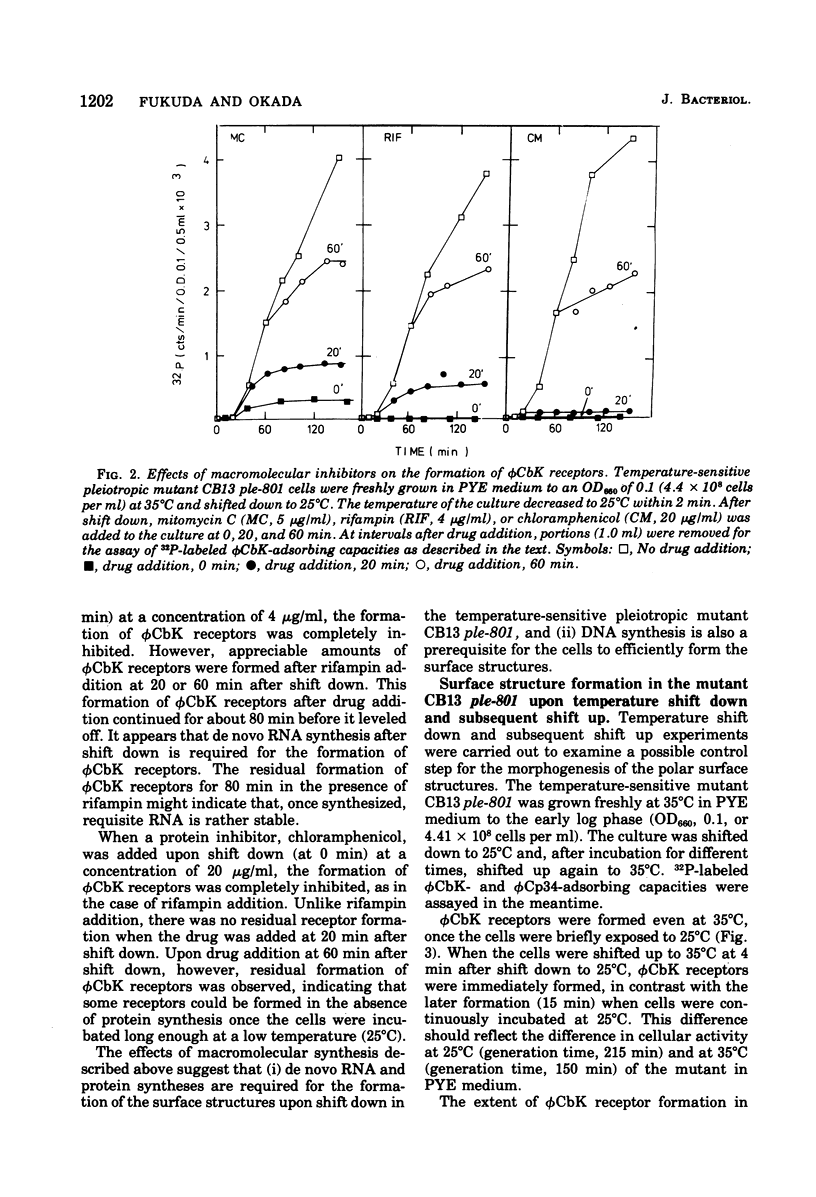
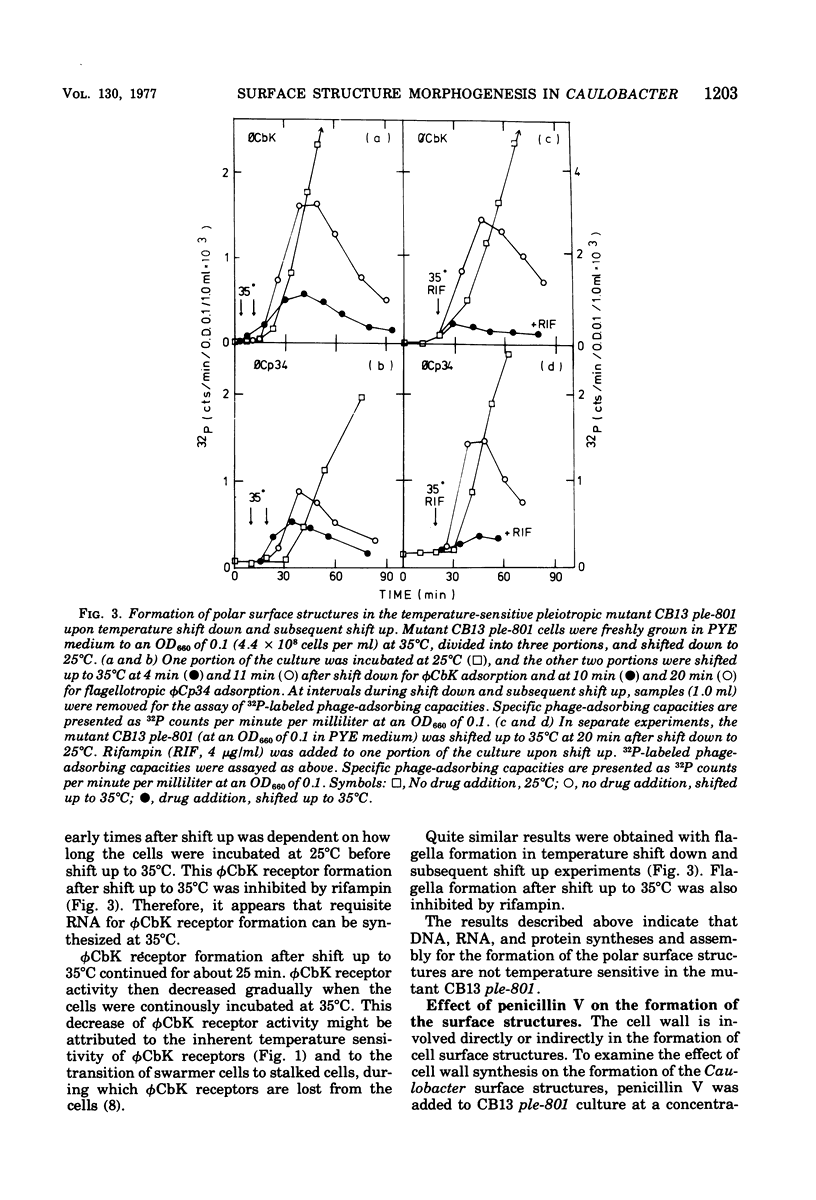
The Caulobacter polar surface structures (flagella, pili, and the deoxyribonucleic acid phage φCbK receptors), which are expressed at proximal sites of swarmer cells in a coordinate manner (Shapiro, Annu. Rev. Microbiol., 30:377-407, 1976) could be blocked by a single mutation. The mutant C. crescentus CB13 ple-801 did not form these surface structures when grown at 35°C. Upon shift down to 25°C, the mutant cells initiated the formation of the surface structures. When mitomycin C was added to the mutant culture upon shift down from 35 to 25°C, φCbK receptor formation was inhibited to a minimal level. Rifampin and chloramphenicol completely inhibited φCbK receptor formation when added to the mutant culture upon shift down. Deoxyribonucleic acid as well as ribonucleic acid and protein synthesis seem to be required for the formation of φCbK receptors. Penicillin V also inhibited φCbK receptor formation, indicating the involvement of cell wall synthesis. When the mutant CB13 ple-801 cells were shifted down briefly from 35 to 25°C and then shifted up to 35°C, flagella and φCbK receptors were formed even at 35°C to different extents depending on how long the cells were incubated at 25°C. This formation of the surface structures at 35°C was inhibited by rifampin. From these results, it appears that translation, assembly, or localization processes for the formation of the surface structures are not temperature sensitive at 35°C in the pleiotropic mutant CB13 ple-801. The syntheses of deoxyribonucleic acid and the cell wall do not appear to be temperature sensitive either, since the mutant grows normally at 35°C. It is suggested that there exists a regulatory step that commits the cells to initiate the synthesis of requisite ribonucleic acid for the formation of the polar surface structures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian-Keshishian N., Shapiro L. Stalked bacteria: properties of deoxriybonucleic acid bacteriophage phiCbK. J Virol. 1970 Jun;5(6):795–800. doi: 10.1128/jvi.5.6.795-800.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iba H., Okada Y. A flagellotropic bacteriophage and flagella formation in Caulobacter. Virology. 1976 Jun;71(2):583–592. doi: 10.1016/0042-6822(76)90383-4. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iida H., Okada Y. Regulation of polar surface structures in Caulobacter crescentus: pleiotropic mutations affect the coordinate morphogenesis of flagella, pili and phage receptors. Mol Gen Genet. 1976 Dec 8;149(2):167–173. doi: 10.1007/BF00332885. [DOI] [PubMed] [Google Scholar]
- Jollick J. D., Wright B. L. A flagella specific bacteriophage for caulobacter. J Gen Virol. 1974 Feb;22(2):197–205. doi: 10.1099/0022-1317-22-2-197. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M. Observations on the adsorption of Caulobacter bacteriophages containing ribonucleic acid. J Gen Microbiol. 1966 Nov;45(2):347–353. doi: 10.1099/00221287-45-2-347. [DOI] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]



