Abstract
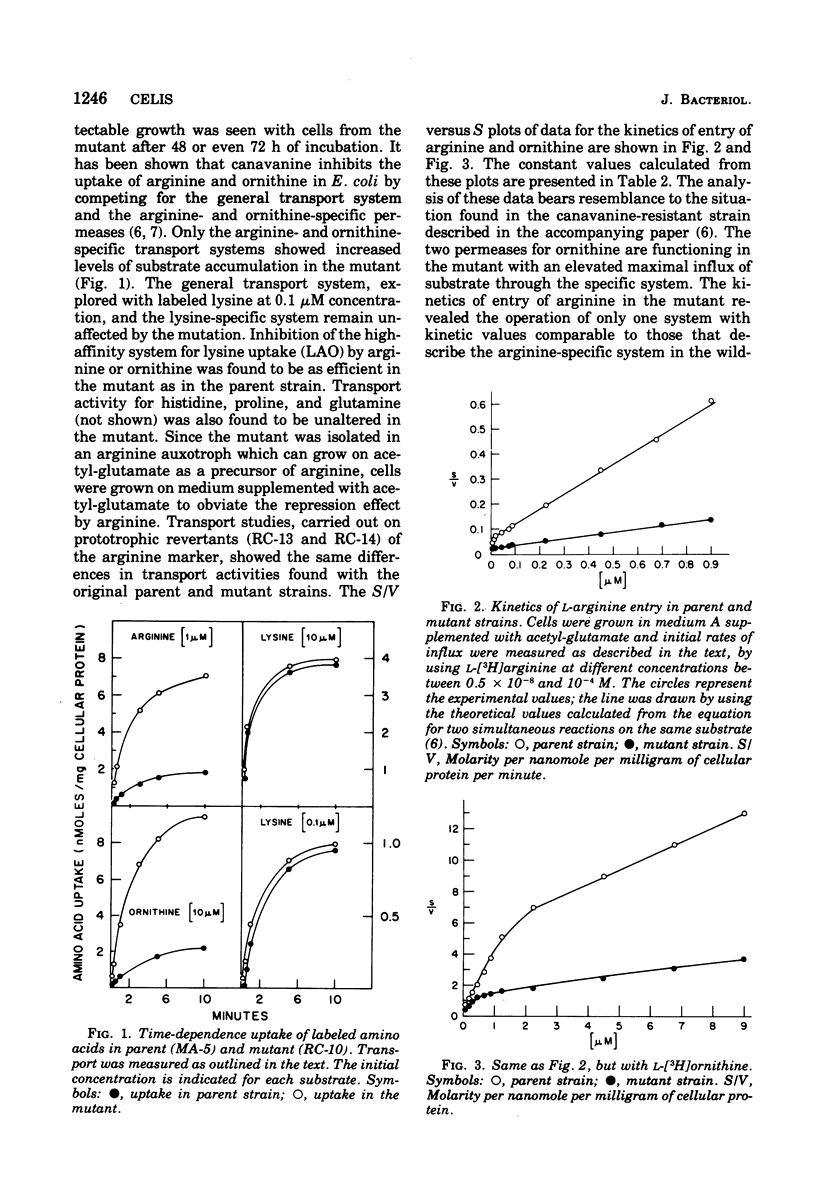
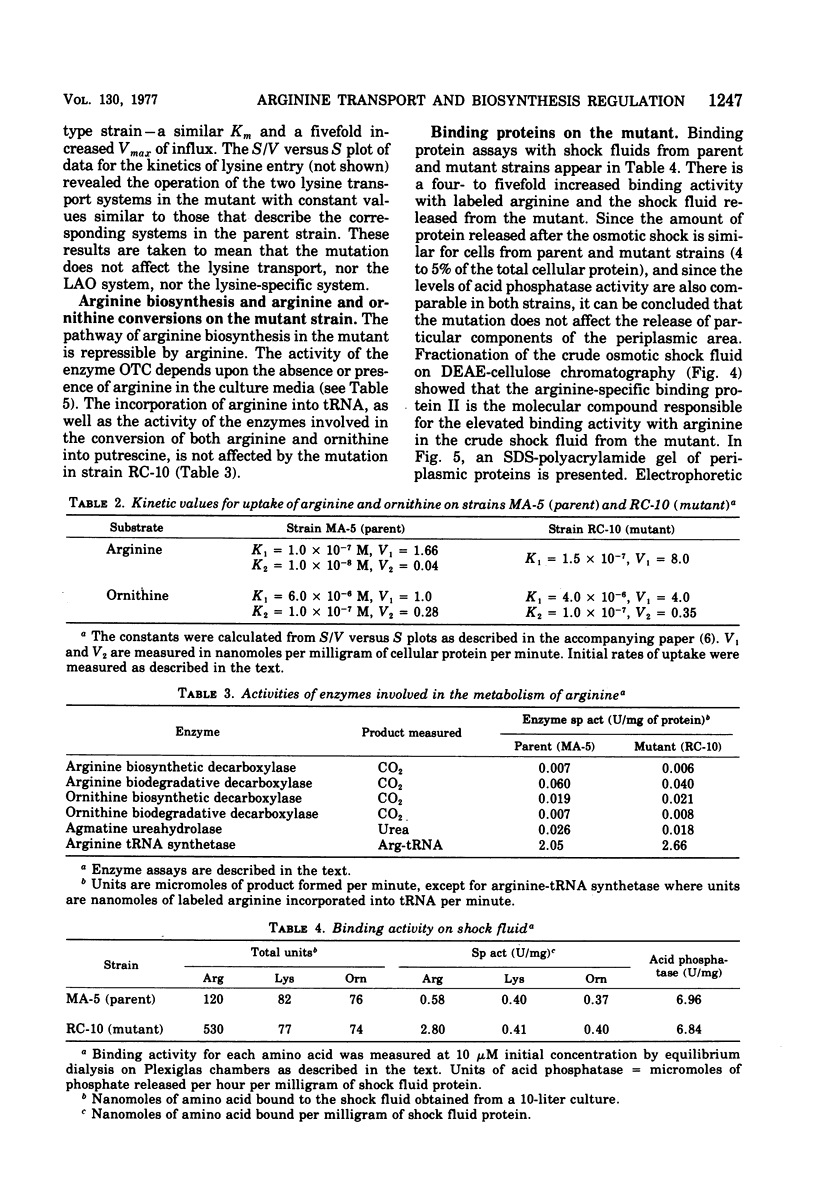
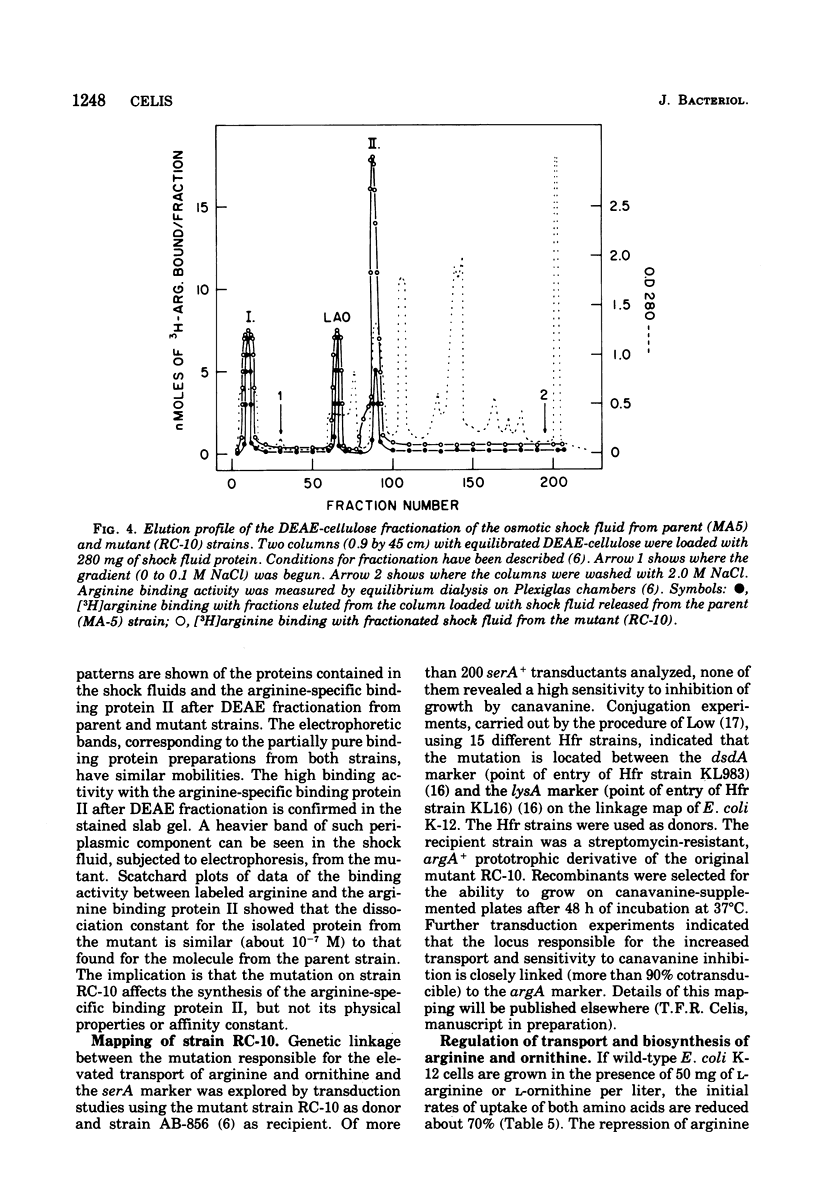
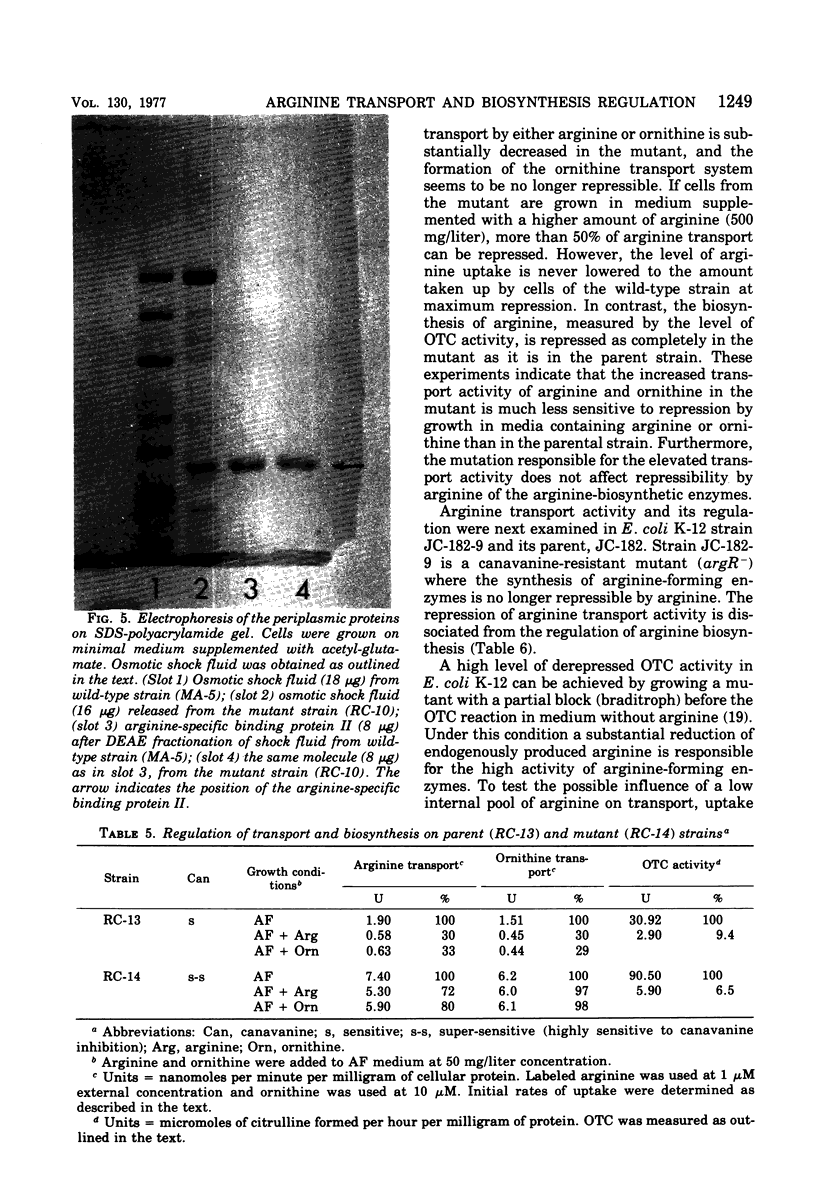
From an arginine auxotrophic strain, a mutant was isolated which is able to utilize d-arginine as a source of l-arginine and shows a high sensitivity to inhibition of growth by canavanine. Transport studies revealed a four- to five-fold increased uptake of arginine and ornithine in cells from the mutant strain. The kinetics of entry of arginine and ornithine evidenced elevated maximal influx values for the arginine- and ornithine-specific transport systems. A close parallel between arginine transport activity and arginine binding activity with one arginine-specific binding periplasmic protein in the mutant strongly suggests that such binding protein is a component of the arginine-specific permease. The affinity between arginine and the binder, isolated from the mutant cells, as well as the electrophoretic mobility of the protein, remain unchanged. The enhanced transport activity of arginine and ornithine with mutant cells is insensitive to repression by arginine or ornithine, whereas the biosynthesis of arginine-forming enzymes is normally repressible. When transport activity was examined in strains with mutations leading to derepression of arginine biosynthesis, the regulation of arginine transport was found to be normal. These studies support the conclusion that arginine transport and arginine biosynthesis, in Escherichia coli K-12, are not regulated in a concerted manner, although both systems may have components in common.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spurich E. N. Protein-protein interaction in transport: periplasmic histidine-binding protein J interacts with P protein. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1877–1881. doi: 10.1073/pnas.73.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W. Structurally defective galactose-binding protein isolated from a mutant negative in the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5414–5424. [PubMed] [Google Scholar]
- Celis T. F. Properties of an Escherichia coli K-12 mutant defective in the transport of arginine and ornithine. J Bacteriol. 1977 Jun;130(3):1234–1243. doi: 10.1128/jb.130.3.1234-1243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis T. F., Rosenfeld H. J., Maas W. K. Mutant of Escherichia coli K-12 defective in the transport of basic amino acids. J Bacteriol. 1973 Nov;116(2):619–626. doi: 10.1128/jb.116.2.619-626.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- Glansdorf N., Sand G. Coordination of enzyme synthesis in the arginine pathway of Escherichia coli K-12. Biochim Biophys Acta. 1965 Oct 11;108(2):308–311. doi: 10.1016/0005-2787(65)90016-x. [DOI] [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. I. The genetic evidence. J Mol Biol. 1969 Jan 14;39(1):73–87. doi: 10.1016/0022-2836(69)90334-9. [DOI] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Walczak W., Klopotowski T. Mutants of Salmonella typhimurium able to utilize D-histidine as a source of L-histidine. J Bacteriol. 1971 Jan;105(1):28–37. doi: 10.1128/jb.105.1.28-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J., Somerville R. L. Mutant strains of Escherichia coli K12 that use D-amino acids. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2484–2487. doi: 10.1073/pnas.68.10.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K., MAAS R., WIAME J. M., GLANSDORFF N. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. I. DOMINANCE OF REPRESSIBILITY IN ZYGOTES. J Mol Biol. 1964 Mar;8:359–364. doi: 10.1016/s0022-2836(64)80199-6. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L., Tsuyumu S., Umbarger H. E. Separate regulation of transport and biosynthesis of leucine, isoleucine, and valine in bacteria. J Bacteriol. 1975 Jun;122(3):994–1000. doi: 10.1128/jb.122.3.994-1000.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Guzman R., Rotman B. Roles of individual mgl gene products in the beta-methylgalactoside transport system of Escherichia coli K12. J Biol Chem. 1976 May 25;251(10):3112–3116. [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]




