Abstract
Increasing resistance of Plasmodium falciparum malaria parasites to chloroquine and the dihydrofolate reductase (DHFR) inhibitors pyrimethamine and cycloguanil have sparked renewed interest in the antimalarial drugs WR99210 and proguanil, the cycloguanil precursor. To investigate suggestions that WR99210 and proguanil act against a target other than the reductase moiety of the P. falciparum bifunctional DHFR–thymidylate synthase enzyme, we have transformed P. falciparum with a variant form of human DHFR selectable by methotrexate. Human DHFR was found to fully negate the antiparasitic effect of WR99210, thus demonstrating that the only significant action of WR99210 is against parasite DHFR. Although the human enzyme also resulted in greater resistance to cycloguanil, no decrease was found in the level of susceptibility of transformed parasites to proguanil, thus providing evidence of intrinsic activity of this parent compound against a target other than DHFR. The transformation system described here has the advantage that P. falciparum drug-resistant lines are uniformly sensitive to methotrexate and will complement transformation with existing pyrimethamine-resistance markers in functional studies of P. falciparum genes. This system also provides an approach for screening and identifying novel DHFR inhibitors that will be important in combined chemotherapeutic formulations against malaria.
The development and wide use of synthetic antimalarial drugs in the latter half of the 20th century has been accompanied by the rapid genesis and spread of drug-resistant strains of the deadliest of human malaria species, the apicomplexan protozoan Plasmodium falciparum. Among the most serious losses are chloroquine and the dihydrofolate reductase (DHFR, EC 1.5.1.3) inhibitors pyrimethamine and cycloguanil, the latter being the active metabolite of proguanil. DHFR catalyzes the NADPH-dependent reduction of dihydrofolate to tetrahydrofolate, an essential cofactor in de novo nucleotide synthesis. In the case of the DHFR inhibitors, several point mutations in the reductase moiety of the bifunctional P. falciparum DHFR–thymidylate synthase (DHFR-TS) enzyme have been linked to different profiles of resistance against pyrimethamine or cycloguanil (1–5). These findings have led to the suggestion that drug-resistant strains might be countered by combinations of alternative DHFR inhibitors (4, 6–8).
One promising antimalarial compound is the dihydrotriazine WR99210, an antifolate that has been found to be effective against P. falciparum in vitro at exquisitely low concentrations (in the nano- to picomolar range) (9, 10). Although early clinical trials revealed poor absorption and tolerance (11), the reduction of side effects by administration of a pro-drug [PS-15 (12)] and the potency of this drug on the opportunistic pathogens Pneumocystis carinii (13), Toxoplasma gondii (14), and Mycobacterium avium complex (15) have led to renewed interest in its use.
In marked contrast to the clear evidence for the action of pyrimethamine against P. falciparum DHFR, data from various studies have suggested that WR99210 might hit another target in addition to or instead of this enzyme (16). Inhibition studies demonstrated that although WR99210 resulted in depletion of dTTP pools (consistent with inhibition of DHFR), addition of 5-formyl tetrahydrofolate (a source of reduced folate) with drug neither restored dTTP levels nor readily attenuated the effects of WR99210, leading to the proposal that this drug was acting on an alternative enzyme involved in the folate synthesis and metabolism pathway (17). In a separate study in which DHFR-deficient yeast were transformed with different variants of P. falciparum DHFR, relative differences in the levels of susceptibility to WR99210 were maintained between these variants in both yeast and P. falciparum (18). However, the IC50 values of this drug were up to 10-fold higher in the transformed yeast, leading to the proposal that a second target present in P. falciparum had not been brought over in the transformation (18). The possibility of a second target has also been thought to explain the slow and difficult appearance of resistance to WR99210 in animal models (19) and the fact that WR99210 retains full potency on lines resistant to pyrimethamine or cycloguanil (9, 10).
A related question has also emerged in studies of proguanil, used since the 1940s to treat falciparum and vivax malaria and now formulated in combination with the electron transport inhibitor atovaquone as the new drug Malarone (20). Proguanil is metabolized to cycloguanil in the liver principally by the hepatic cytochrome P450 isoenzyme CYP2C19 (21). Although it is widely assumed that the effect of proguanil is due solely to activity of the cycloguanil metabolite, and several studies argue strongly that cycloguanil acts upon DHFR (4, 5, 8), early reports described an intrinsic activity of proguanil separate from cycloguanil, suggesting inhibition of a separate target. In addition, proguanil was found to be equally effective in vitro on lines of P. falciparum that were either resistant or sensitive to cycloguanil (22). When tested in humans or simian models, proguanil was found to be 2- to 4-fold more active than the same concentration of cycloguanil (23, 24), with subsequent studies demonstrating that this was not due to differences in rates of metabolism, indicating that a significant part of the antimalarial activity resided in the parent compound (25).
The unambiguous identification and characterization of the targets of WR99210 and proguanil has important and clear implications for the development and testing of new antimalarial drugs. To directly investigate the role of DHFR in the action of these drugs, we have transformed P. falciparum with a human DHFR sequence that is innately resistant to antimalarial agents and confers resistance to the folate analog methotrexate (MTX). This report relates the findings from these experiments and discusses their implications for drug screening and combination chemotherapy.
MATERIALS AND METHODS
Plasmid Constructs.
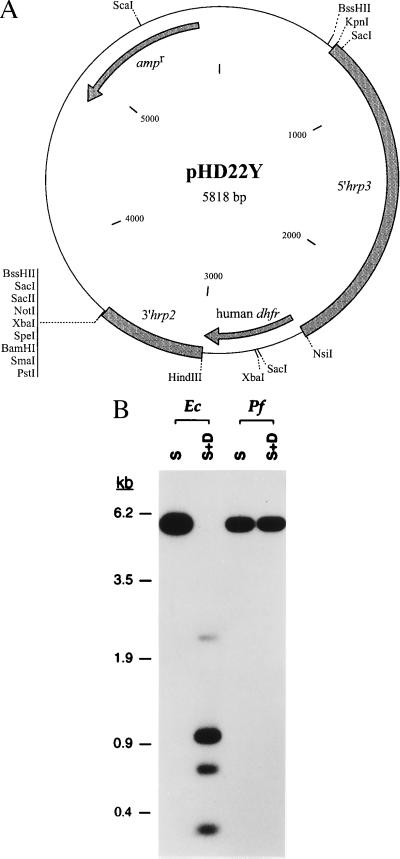
The human dhfr gene was amplified from a cDNA clone (26) using PCR with the primers 5′-cctttttatgcatggttcgctaaactgcatcg and 3′-aatttcaagcttaatcattcttctcatatacttc. This cDNA encodes the introduced mutation Leu → Tyr at residue 22 located within the active site (27). This mutation greatly decreases the affinity of MTX for the apoenzyme in the presence of dihydrofolate and NADPH (Ki increase of 3,200 relative to wild-type enzyme) while leading to only minimal loss in catalytic efficiency at physiological pH (26). The vector pHD22Y (see Fig. 2A) was generated by inserting the human dhfr gene (as a NsiI–HindIII fragment) in the place of the luciferase gene in the pHLH-1 construct (28). Expression occurs under the control of the flanking P. falciparum regulatory elements 5′hrp3 and 3′hrp2 (28).
Figure 2.
(A) Map of the P. falciparum transfection vector pHD22Y expressing the highly MTX-resistant human L22Y dhfr allele under regulatory control of the P. falciparum elements 5′-hrp3 and 3′-hrp2 (28). (B) Detection of pHD22Y DNA replicated as episomes in transfected P. falciparum. T1+MTX genomic DNA (prepared 2 months posttransfection) and DNA from a representative plasmid rescued from T1+MTX were restricted with ScaI (S; which cuts once in the vector) or ScaI + DpnI (S+D; with over a dozen recognition sites) and hybridized with a probe corresponding to the pBluescriptII SK(+) vector backbone. Although P. falciparum-replicated episomes were resistant to DpnI digestion, digestion of E. coli-replicated plasmids resulted in detection of bands of the expected sizes 2.3, 1.0, 0.7, and 0.3 kb (the largest being fainter as the majority of this fragment contains 5′-hrp3 sequences).
P. falciparum Transfection, Selection of Transformants, and DNA Analysis.
P. falciparum parasites (FCB strain) were cultivated as described (29). Transfection of parasites was as previously reported (28), except for adjustments in the electroporator settings to improve the delivery of supercoiled DNA. These adjustments were made based on results from transfection experiments with the luciferase reporter construct pHLH-1 (28). Immediately after transfection, electroporated samples were mixed with 10 ml of culture medium [RPMI 1640 with l-glutamine (catalogue no. 31800, Life Technologies, Gaithersburg, MD), 50 mg/liter hypoxanthine (Sigma), 25 mM Hepes (ultrol grade, Calbiochem), 0.225% NaHCO3 (Biofluids, Rockville, MD), 0.5% Albumax I (Life Technologies), and 10 μg/ml gentamycin (Life Technologies)] and grown at 37°C in tissue culture flasks gassed with 5% CO2, 5% O2, and 90% N2. For the luciferase assays, parasites were harvested by centrifugation 72 hr later and each culture was lysed for 5 min at room temperature with 0.5 ml 1× PBS (pH 7.4) containing 0.15% saponin. A total of 10 ml 1× PBS was then added and the sample spun 10 min at 10,000 × g. Following removal of the supernatant and erythrocyte ghosts, the parasite pellet was washed once with 1.0 ml 1× PBS and transferred to a 1.5-ml Eppendorf tube. After recentrifugation (5 min at 13,000 × g), remaining ghosts were removed and the pellet was gently resuspended in 50 μl 1× lysis buffer (Promega). To each luminometer cuvette was added 100 μl luciferin substrate (Promega) at room temperature. Luciferase activity was measured 30–60 sec following the addition of 20 μl of parasite lysate to the cuvette. Initial experiments with low voltage, high capacitance conditions showed high luciferase activity with 0.3–0.32 kV, 960 μF, with a steep decrease in signal at lower voltages (data not shown). The settings 0.31 kV, 960 μF (used with 0.2-cm cuvettes) were subsequently chosen for comparison with the previous values of 2.5 kV, 25 μF (ref. 28; employing 0.4-cm cuvettes). The same cell numbers and DNA quantities were transfected in the 0.4-cm cuvette (one sample) and 0.2-cm cuvettes (two transfections of half the sample each). The low voltage, high capacitance setting was found to increase expression signals in transfected parasites by 4- to 10-fold (Table 1).
Table 1.
Luciferase activities of P. falciparum parasite cultures transfected with the reporter construct pHLH-1 using high versus low voltage settings
| Exp. | Voltage/capacitance | Luciferase activity* |
|---|---|---|
| 1 | 2.5 kV, 25 μF | 0.91 |
| 0.31 kV, 960 μF | 3.69 | |
| 2 | 2.5 kV, 25 μF | 3.43 |
| 0.31 kV, 960 μF | 16.60 | |
| 3 | 2.5 kV, 25 μF | 0.64 |
| 0.31 kV, 960 μF | 6.19 | |
| 4 | 2.5 kV, 25 μF | 0.19 |
| 0.31 kV, 960 μF | 0.73 |
Calculated from duplicate transfected cultures. The wide variation in signal between experiments reflects differences in the quantity of DNA (20–65 μg), the initial parasitemia (3–12%), and the parasite stages predominating in the cultures at the time of transfection.
P. falciparum genomic DNA was prepared from 25–100 ml of culture at ≥5% schizonts as described (30). Rescue of plasmids into Escherichia coli was performed by electroporating 100 ng of purified P. falciparum genomic DNA into SURE cells (Stratagene) and plating out on Luria–Bertani broth plus 100 μg/ml ampicillin. For Southern hybridization analysis, plasmid and P. falciparum genomic DNA were digested to completion and 2 μg and 0.2 ng, respectively, were loaded onto a 0.8%, 30-cm agarose gel and run overnight in 1× TAE buffer at 45 V. Following positive pressure (PosiBlot; Stratagene) transfer onto NylonPlus (Schleicher & Schuell) and prehybridization, the blot was hybridized overnight at 60°C with linearized, 32P-labeled pBS vector and washed up to a final stringency of 0.3× SSPE/0.5% SDS at 62°C. The blot was exposed to x-ray film (Kodak Bio-Max) for 30 min at −70°C.
Drug Response Assays.
MTX (tissue culture grade) was purchased from Sigma. WR99210 [BRL 6231 (31)], proguanil (chlorguanide), and cycloguanil were obtained from W. Milhous and D. Kyle (Walter Reed Army Institute of Research, Washington, DC). All drugs were dissolved in dimethyl sulfoxide at 5–10 mg/ml and stored at −80°C. Working solutions (made in complete medium) were kept at 4°C and renewed every week. Drug susceptibilities were determined by the method of Thaithong and Beale (32). Parasites were cultured at a starting parasitemia of 0.2–0.4% in 96-well tissue culture plates and exposed to drug for 72 hr, with one change of medium at 48 hr. Giemsa-stained smears were made at the end of the 72-hr incubation. Parasitemia was calculated after counting a total of at least 2,000 red blood cells (RBC) or, in the case of parasitemia <0.2%, examining 25–30 fields of 150–100 RBC/field. Percentage inhibition of growth and inhibition constants (IC) were calculated by comparison with parasitemia determined from ≥4,000 cells counted in two control wells without drug.
RESULTS
Uniform Susceptibility to MTX in P. falciparum Isolates Expressing Known DHFR Variants.
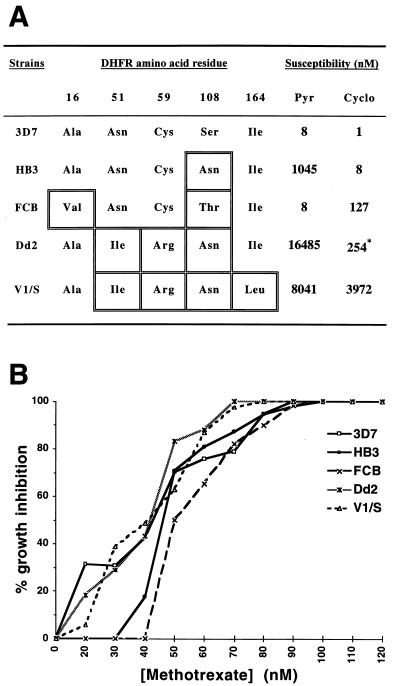
To test the possible effect of P. falciparum DHFR point mutations on susceptibility to MTX [a powerful DHFR inhibitor (33)], we performed drug assays on representative parasite lines chosen to cover the set of substitutions reported at five different positions in this enzyme (4, 5) (Fig. 1A). Results showed no differences in levels of susceptibility, with 50–83% inhibition at 50 nM and 100% inhibition at 100 nM (Fig. 1B). These values were reproduced in tests on FCR-3 and 3B-D5 whose dhfr sequences match those of FCB and HB3, respectively (data not shown).
Figure 1.
(A) Pyrimethamine and cycloguanil susceptibilities of P. falciparum lines containing different point mutations in the DHFR sequence (ref. 4 and unpublished data). ∗, Value reflects folate and p-aminobenzoic acid (PABA) antagonism of the effect of cycloguanil on Dd2 (4). (B) Growth inhibition curves of the five different parasite lines as a function of MTX concentration.
Transformation of P. falciparum Erythrocyte Stages with an Episomally Replicating DNA Encoding a Human DHFR Sequence.
The transfection vector pHD22Y expresses a human DHFR sequence [containing the highly MTX-resistant L22Y substitution (26)] under the regulatory control of P. falciparum promoter and terminator sequences (Fig. 2A). Following electroporation of this vector into P. falciparum, the culture was split into two lines (T1+MTX and T2+MTX) and MTX (2.2 μM and 8.8 μM, respectively) was added 48 hr later. Parasites were detected by microscopy at days 16–18 and subsequently maintained at a MTX concentration of 8.8 μM. These high MTX levels prevented selection of nontransfected MTX-resistant mutants. Control cultures electroporated with equivalent amounts of the control vector pHRPCAT (28) and maintained under the same MTX levels for as long as 50 days posttransfection showed no parasite growth.
Plasmid rescue performed on T1+MTX and T2+MTX genomic DNA prepared 1 and 2 months after transfection yielded comparable numbers of E. coli transformants (1–1.5 × 105 cfu/μg of genomic DNA), indicating that episomes were maintained in the transfected cultures. Restriction enzyme and PCR analyses of a dozen plasmid clones from each rescued sample showed no DNA rearrangements compared with the pretransfection pHD22Y plasmid (data not shown). To demonstrate replication of the episomes by P. falciparum, we employed the restriction enzyme DpnI, which depends on DNA methylation for cleavage and therefore cuts DNA replicated by E. coli but not by P. falciparum (34). Results showed lack of DpnI cleavage of plasmid sequences present in the T1+MTX parasite genomic DNA preparation, whereas this enzyme produced multiple fragments from E. coli-replicated pHD22Y DNA, indicating that the transfected plasmid DNA had been replicating episomally in P. falciparum (Fig. 2B). For both sources of replicated DNA, ScaI digestion alone gave the expected size of the linearized 5.8-kb construct. Identical results were obtained with DNA from the T2+MTX line (data not shown).
MTX Resistance of P. falciparum Transformed by pHD22Y.
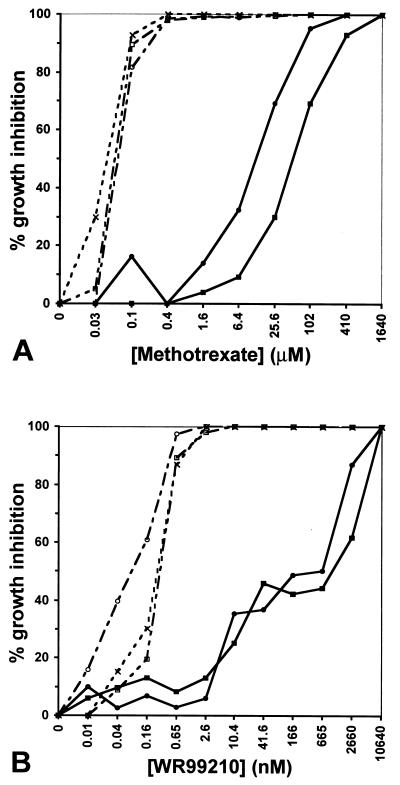
MTX drug assays on lines T1+MTX and T2+MTX transformed with the pHD22Y plasmid showed IC90 values of 100 μM and 400 μM, over 4,000-fold higher than the control value of 0.1 μM found for the nontransfected parental strain FCB (Fig. 3A). The killing curve for the episomally transformed lines extended to even higher concentrations, as viable ring-stage T2+MTX parasites were found to grow even at 1,100 μM. To confirm that increased resistance was due to episomal replication of the human dhfr vector, parallel tests were conducted on the lines T1-MTX and T2-MTX, which 1 month earlier had been split from the transformed lines and allowed to propagate in the absence of drug. This regimen results in loss of episomes from the parasites due to outgrowth of daughter progeny lacking these elements (34–36). Without MTX pressure the pHD22Y episomes were lost and the MTX-resistant populations were overgrown by parasites that had reverted to a fully sensitive phenotype.
Figure 3.
Inhibition levels of transfected versus control parasites as a function of (A) MTX and (B) WR99210 concentration, shown in logarithmic scale (log4). These assays were performed on three separate occasions, with similar results. --×--, FCB; —•—, T1+MTX; —○–-, T1-MTX; —▪—, T2+MTX; —□–-, T2-MTX. Lines T1+MTX and T2+MTX had been maintained 65 days under MTX pressure whereas lines T1-MTX and T2-MTX were grown for 39 days under pressure and then a further 26 days (13 generations) without MTX.
Human DHFR Complements the Reductase Function of P. falciparum DHFR-TS and Fully Negates the Antiparasitic Effect of WR99210.
Transfected and control parasites were compared for their response to the experimental antimalarial compound WR99210 (Fig. 3B). This drug was found to exert a profound effect on nontransformed parasites at 0.65 nM (87% inhibition) and was fully effective at 2.6 nM, consistent with values previously reported for the FCB strain (10). In contrast, 2,660 nM was required to produce 87% and 62% inhibition in the T1+MTX and T2+MTX lines containing the pHD22Y episomes. Even at concentrations >5,000 nM, viable transformed parasites were detected (data not shown). Full sensitivity to WR99210 was completely restored in parasites that had lost the episomes following removal of MTX pressure.
Evidence that Proguanil Itself Acts on a Target Separate from DHFR.
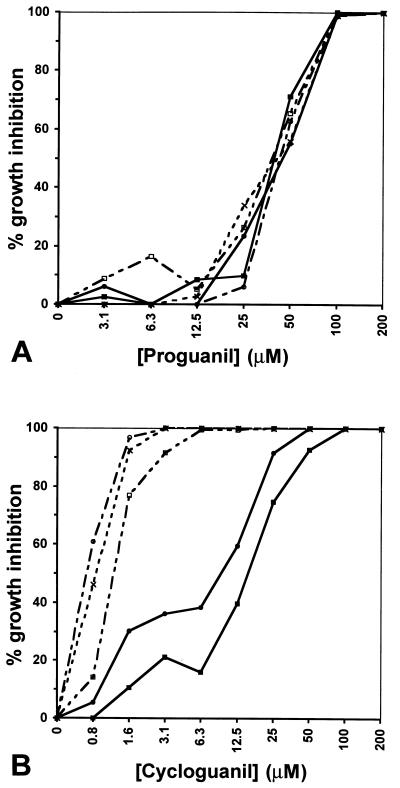
Transformed parasites expressing the human DHFR sequence were found to have no decrease in their level of susceptibility to proguanil (Fig. 4A). Inhibition profiles were the same for all cultures tested (transformed and nontransformed controls): 50 μM resulted in inhibition levels of 55–71% whereas 100 μM resulted in >98% inhibition. This finding for proguanil was in marked contrast to the cycloguanil response, which was decreased in the transformed parasites (Fig. 4B). Indeed, whereas 1.6 μM of cycloguanil inhibited 77–97% of the control parasites FCB, T1-MTX, and T2-MTX, similar inhibition (75–92%) was only seen at 25 μM in the episomally transfected lines (T1+MTX and T2+MTX) and up to 100 μM was needed to obtain full inhibition of the T2+MTX line. We can discount the possibility that the effect of proguanil was due to any conversion into the metabolite cycloguanil as otherwise an increase in resistance to proguanil would have been observed in the transformed parasites, and previous data has shown lack of metabolism of proguanil to cycloguanil in vitro (22). In the transformed parasites, the 20- to 40-fold reduction in susceptibility to cycloguanil, compared with the 4,000-fold reduction for MTX, is presumably explained by the presence of the paired A16V and S108T mutations in the dhfr locus that reduce binding of cycloguanil to the parasite enzyme (8).
Figure 4.
Effect of (A) proguanil and (B) cycloguanil on human DHFR-transfected versus nontransfected control parasites. Inhibition levels are shown as a function of drug concentration, shown in logarithmic scale (log2). These results were confirmed on three separate occasions. --×--, FCB; —•—, T1+MTX; —○–-, T1-MTX; —▪—, T2+MTX; —□–-, T2-MTX. The inhibitory concentrations required for cycloguanil on the parental strain FCB closely matched those recorded for this same strain by Childs and Lambros (10), though the concentrations are 10-fold higher than those determined by Peterson et al. (4). Sequencing of the dhfr loci confirmed identity between FCB used in this study and that previously described (4).
DISCUSSION
In the development of antimalarial agents there have been important and unresolved questions regarding the targets and mechanisms of action of two compounds presumed to affect the folate pathway. For both of these compounds, WR99210 and proguanil, various lines of evidence have suggested the chemotherapeutic action was directed against one or more targets independent of the DHFR enzyme. Using the DHFR inhibitor MTX, we have selected transformed lines of P. falciparum in which the parasite DHFR activity is supplemented by a MTX-resistant human DHFR. Transformed parasites showed a 4,000-fold increase in resistance to both MTX and WR99210. This result demonstrates that the action of WR99210 must be against parasite DHFR and is consistent with previous data indicating the excellent therapeutic window of this drug (37). Significant inhibition of a second target in P. falciparum by WR99210 is not supported by these results.
WR99210 shows no cross-resistance with pyrimethamine or cycloguanil (refs. 10 and 18; our unpublished results); in fact one study reported that pyrimethamine-resistant cycloguanil-sensitive parasites display collateral hypersensitivity to WR99210 (18). In vivo studies in Aotus monkeys have also revealed that WR99210 and its pro-drug (which are rapidly eliminated, possibly favorable for reducing selection pressure on inducing resistance) are far more potent than proguanil or cycloguanil (12). Recently, an in vitro study on alternatives to pyrimethamine-sulfadoxine (Fansidar; Hoffman–La Roche) found the greatest synergistic potency with WR99210 and the dihydropteroate synthase inhibitor dapsone, their inhibitory concentrations being 2–3 orders of magnitude lower than those required for pyrimethamine-sulfadoxine (37). The efficacy of WR99210 against opportunistic pathogens (13–15, 40) and the synergistic effects of combining this drug with sulfa compounds (38, 39) should help promote pharmaceutical interest in WR99210 and may provide an effective addition to the antimalarial pharmacopoeia.
Proguanil contrasts with both cycloguanil and WR99210 in that the level of susceptibility is unaltered in human DHFR-transformed parasite lines. These results suggest that proguanil has an intrinsic activity independent of DHFR, an activity that may be an alternative target for the development of antimalarial drugs. Our data help to explain previous findings that proguanil can be equally effective on cycloguanil-resistant or -sensitive parasites and is considerably more effective in monkeys than the equivalent dose of cycloguanil (22, 23, 25). Watkins et al. (22), however, calculated that the concentrations required for parasite inhibition by the parent compound proguanil exceed levels achievable by standard therapeutic doses and thus proposed that the clinical activity of the drug is due principally to the cycloguanil metabolite. This apparent paradox may be resolved by the recent report that proguanil is concentrated in red blood cells (12).
An alternative explanation for the unchanged proguanil response of the transformed parasites is that the parasite and human enzymes could be equally affected by this drug, thereby conferring no increase in resistance from the presence of human DHFR. We consider this unlikely because proguanil has a high therapeutic window with no serious toxicity even after long-term prophylaxis or administration of exceedingly high doses [up to 1,400 mg daily (41)]. Indeed, studies have shown that some individuals convert proguanil to cycloguanil very poorly (16), with one large study in Tanzania revealing that the proguanil/cycloguanil ratio varied from 0.8 to 249 (42). Poor metabolizers of proguanil can account for up to 70% of the population in endemic areas (43). Importantly, several studies have indicated that prophylactic failure following proguanil administration is no more frequent in these poor metabolizers than in extensive metabolizers that actively convert proguanil to cycloguanil (44–46). Equivalent efficacy of proguanil in poor versus extensive metabolizers has recently been confirmed in a large prophylactic study in Vanuatu (A. Kaneko, personal communication). These clinical data complement and strengthen our transformation results, indicating an intrinsic activity of proguanil that may act on a target separate from DHFR. Proguanil in combination with new compounds such as atovaquone may therefore prove to be an efficient therapeutic tool even in regions where poor metabolizers are frequent or where resistance to cycloguanil already exists.
In apicomplexan and kinetoplastid protozoa, DHFR resides on the same polypeptide chain as TS. These two enzymes catalyze sequential reactions in the biosynthesis of dTMP. Evidence for channeling of the dihydrofolate product from the TS moiety to DHFR (47) is thought to support the proposal that physical association and coordinated expression of these two enzymes conferred an evolutionary advantage resulting in fixation of the bifunctional dhfr-ts gene (48). While such a selection advantage may exist, our results indicate that the noncoupled human DHFR enzyme is able to provide the parasite with sufficient tetrahydrofolate pools at concentrations of MTX (or WR99210) that block the function of the parasite DHFR moiety, thus showing that physical association of these two crucial enzymes is not a necessity for the parasite. We also note that complementation of the parasite enzyme by the human DHFR selectable marker provides the means to genetically manipulate parasites already resistant to pyrimethamine. Given the uniform susceptibility of P. falciparum DHFR variants to MTX, this system will be broadly applicable to in vitro transformation of drug-resistant or -sensitive P. falciparum.
Parasites transformed with the human enzyme provide a potentially powerful resource in the search for DHFR inhibitors that are effective against the various mutant forms of P. falciparum DHFR. WR99210 is an example of an inhibitor that acts with very high and comparable efficiency against known mutant forms of the parasite enzyme while leaving the human enzyme unaffected. Numerous triazines, diaminoquinazolines, diaminopyrimidines, and pyrrolopyrimidines have been described (49, 50) and some of these have been shown to be effective against forms of parasite DHFR-TS expressed in E. coli or yeast (18, 50, 51). Using P. falciparum transformed with the human enzyme will now facilitate assessment of the relative activities of novel lead compounds against parasite and host DHFR. Such a system can be expanded to incorporate parasites differing in their drug-resistant profiles and cloned transformants in which the human enzyme sequence is integrated into the parasite genome.
Acknowledgments
We are grateful to Anna Liu for technical help. We thank Dr. Raymond Blakley for the human dhfr cDNA clone; Dennis Kyle and Wilbur Milhous for the antimalarial agents; Kirk Deitsch and Osamu Kaneko for discussions; and Akira Kaneko for sharing unpublished results.
ABBREVIATIONS
- DHFR-TS
dihydrofolate reductase–thymidylate synthase
- MTX
methotrexate
Note
Since preparation of this manuscript we have identified integration of the pHD22Y construct into a targeted region of the parasite genome. This removes the inherent instability of episomes in the transformed lines and provides stable lines that will facilitate the screening of novel DHFR inhibitors.
References
- 1.Peterson D S, Walliker D, Wellems T E. Proc Natl Acad Sci USA. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowman A F, Morry M J, Biggs B A, Cross G A, Foote S J. Proc Natl Acad Sci USA. 1988;85:9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zolg J W, Plitt J R, Chen G X, Palmer S. Mol Biochem Parasitol. 1989;36:253–262. doi: 10.1016/0166-6851(89)90173-4. [DOI] [PubMed] [Google Scholar]
- 4.Peterson D S, Milhous W K, Wellems T E. Proc Natl Acad Sci USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foote S J, Galatis D, Cowman A F. Proc Natl Acad Sci USA. 1990;87:3014–3017. doi: 10.1073/pnas.87.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirawaraporn W, Sirawaraporn R, Cowman A F, Yuthavong Y, Santi D V. Biochemistry. 1990;29:10779–10785. doi: 10.1021/bi00500a009. [DOI] [PubMed] [Google Scholar]
- 7.Wellems T E. Parasitol Today. 1991;7:110–112. doi: 10.1016/0169-4758(91)90168-n. [DOI] [PubMed] [Google Scholar]
- 8.Sirawaraporn W, Sathitkul T, Sirawaraporn R, Yuthavong Y, Santi D V. Proc Natl Acad Sci USA. 1997;94:1124–1129. doi: 10.1073/pnas.94.4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieckmann K H. WHO Tech Rep Ser. 1973;529:58. [Google Scholar]
- 10.Childs G E, Lambros C. Ann Trop Med Parasitol. 1986;80:177–181. doi: 10.1080/00034983.1986.11812002. [DOI] [PubMed] [Google Scholar]
- 11.Canfield, C. J. (1986) in Chemotherapy of Malaria, Monograph Series, ed. Bruce-Chwatt, L. J. (World Health Organization, Geneva), Vol. 27, pp. 99–100.
- 12.Canfield C J, Milhous W K, Ager A L, Rossan R N, Sweeney T R, Lewis N J, Jacobus D P, Hadiputranto H, Larasati R P, Pudjoprawoto N, Subianto B, Hoffman S L. Am J Trop Med Hyg. 1993;49:121–126. doi: 10.4269/ajtmh.1993.49.121. [DOI] [PubMed] [Google Scholar]
- 13.Hughes W T, Jacobus D P, Canfield C, Killmar J. Antimicrob Agents Chemother. 1993;37:1417–1419. doi: 10.1128/aac.37.7.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brun-Pascaud M, Chau F, Garry L, Jacobus D, Derouin F, Girard P M. Antimicrob Agents Chemother. 1996;40:2067–2070. doi: 10.1128/aac.40.9.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer S C, Majumder S K, Cynamon M H. Antimicrob Agents Chemother. 1995;39:1862–1863. doi: 10.1128/aac.39.8.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters W. Chemotherapy and Drug Resistance in Malaria. London: Academic; 1987. [Google Scholar]
- 17.Yeo A E, Seymour K K, Rieckmann K H, Christopherson R I. Ann Trop Med Parasitol. 1997;91:17–23. doi: 10.1080/00034983.1997.11813107. [DOI] [PubMed] [Google Scholar]
- 18.Wooden J M, Hartwell L H, Vasquez B, Sibley C H. Mol Biochem Parasitol. 1997;85:25–40. doi: 10.1016/s0166-6851(96)02808-3. [DOI] [PubMed] [Google Scholar]
- 19.Knight D J, Mamalis P, Peters W. Ann Trop Med Parasitol. 1982;76:1–7. [PubMed] [Google Scholar]
- 20.Looareesuwan S, Viravan C, Webster H K, Kyle D E, Hutchinson D B, Canfield C J. Am J Trop Med Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 21.Wright J D, Helsby N A, Ward S A. Br J Clin Pharmacol. 1995;39:441–444. doi: 10.1111/j.1365-2125.1995.tb04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins W M, Sixsmith D G, Chulay J D. Ann Trop Med Parasitol. 1984;78:273–278. doi: 10.1080/00034983.1984.11811816. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt L H, Loo T L, Fradkin R, Hughes H B. Proc Soc Exp Biol Med. 1952;80:367–370. doi: 10.3181/00379727-80-19625. [DOI] [PubMed] [Google Scholar]
- 24.Robertson G I. Trans R Soc Trop Med Hyg. 1957;51:488–492. doi: 10.1016/0035-9203(57)90036-6. [DOI] [PubMed] [Google Scholar]
- 25.Smith C C, Ihrig J, Menne R. Am J Trop Med Hyg. 1961;10:694–703. [Google Scholar]
- 26.Lewis W S, Cody V, Galitsky N, Luft J R, Pangborn W, Chunduru S K, Spencer H T, Appleman J R, Blakley R L. J Biol Chem. 1995;270:5057–5064. doi: 10.1074/jbc.270.10.5057. [DOI] [PubMed] [Google Scholar]
- 27.Matthews D A, Alden R A, Bolin J T, Freer S T, Hamlin R, Xuong N, Kraut J, Poe M, Williams M, Hoogsteen K. Science. 1977;197:452–455. doi: 10.1126/science.17920. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Sifri C D, Lei H H, Su X Z, Wellems T E. Proc Natl Acad Sci USA. 1995;92:973–977. doi: 10.1073/pnas.92.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trager W, Jensen J B. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 30.Wellems T E, Walliker D, Smith C L, do Rosario V E, Maloy W L, Howard R J, Carter R, McCutchan T F. Cell. 1987;49:633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]
- 31.Knight D J. Ann Trop Med Parasitol. 1981;75:1–6. [PubMed] [Google Scholar]
- 32.Thaithong S, Beale G H. Trans R Soc Trop Med Hyg. 1981;75:271–273. doi: 10.1016/0035-9203(81)90333-3. [DOI] [PubMed] [Google Scholar]
- 33.Huennekens F M. Adv Enzyme Regul. 1994;34:397–419. doi: 10.1016/0065-2571(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Kirkman L A, Wellems T E. Proc Natl Acad Sci USA. 1996;93:1130–1134. doi: 10.1073/pnas.93.3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dijk M R, Waters A P, Janse C J. Science. 1995;268:1358–1362. doi: 10.1126/science.7761856. [DOI] [PubMed] [Google Scholar]
- 36.Crabb B S, Cowman A F. Proc Natl Acad Sci USA. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winstanley P A, Mberu E K, Szwandt I S, Breckenridge A M, Watkins W M. Antimicrob Agents Chemother. 1995;39:948–952. doi: 10.1128/aac.39.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott H V, Rieckmann K H, O’Sullivan W J. Trans R Soc Trop Med Hyg. 1987;81:715–721. doi: 10.1016/0035-9203(87)90004-6. [DOI] [PubMed] [Google Scholar]
- 39.Yeo A E, Rieckmann K H. Trop Med Parasitol. 1994;45:136–137. [PubMed] [Google Scholar]
- 40.Shah L M, DeStefano M S, Cynamon M H. Antimicrob Agents Chemother. 1996;40:2644–2645. doi: 10.1128/aac.40.11.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams A R, Maegraith B G, King J D, Townshend R H, Davey T H, Havard R E. Ann Trop Med Parasitol. 1945;39:225–231. [PubMed] [Google Scholar]
- 42.Skjelbo E, Mutabingwa T K, Bygbjerg I, Nielsen K K, Gram L F, Broosen K. Clin Pharmacol Ther. 1996;59:304–311. doi: 10.1016/S0009-9236(96)80008-7. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko A, Kaneko O, Taleo G, Bjorkman A, Kobayakawa T. Lancet. 1997;349:921–922. doi: 10.1016/S0140-6736(05)62696-7. [DOI] [PubMed] [Google Scholar]
- 44.Mberu E K, Wansor T, Sato H, Nishikawa Y, Watkins W M. Trans R Soc Trop Med Hyg. 1995;89:658–659. doi: 10.1016/0035-9203(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 45.Mutabingwa T K, Malle L N, de Geus A, Oosting J. Trop Geogr Med. 1993;45:6–14. [PubMed] [Google Scholar]
- 46.Ward S A, Watkins W M, Mberu E, Saunders J E, Koech D K, Gilles H M, Howells R E, Breckenridge A M. Br J Clin Pharmacol. 1989;27:781–787. doi: 10.1111/j.1365-2125.1989.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meek T D, Garvey E P, Santi D V. Biochemistry. 1985;24:678–686. doi: 10.1021/bi00324a021. [DOI] [PubMed] [Google Scholar]
- 48.Ivanetich K M, Santi D V. FASEB J. 1990;4:1591–1597. doi: 10.1096/fasebj.4.6.2180768. [DOI] [PubMed] [Google Scholar]
- 49.Mamalis P, Werbel L M. In: Antimalarial Drugs. Peters W, Richards W, editors. New York: Springer; 1984. pp. 387–442. [Google Scholar]
- 50.Brobey R K, Sano G, Itoh F, Aso K, Kimura M, Mitamura T, Horii T. Mol Biochem Parasitol. 1996;81:225–237. doi: 10.1016/0166-6851(96)02704-1. [DOI] [PubMed] [Google Scholar]
- 51.Sirawaraporn W, Prapunwattana P, Sirawaraporn R, Yuthavong Y, Santi D V. J Biol Chem. 1993;268:21637–21644. [PubMed] [Google Scholar]