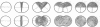Abstract
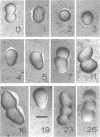
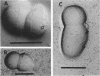
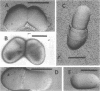
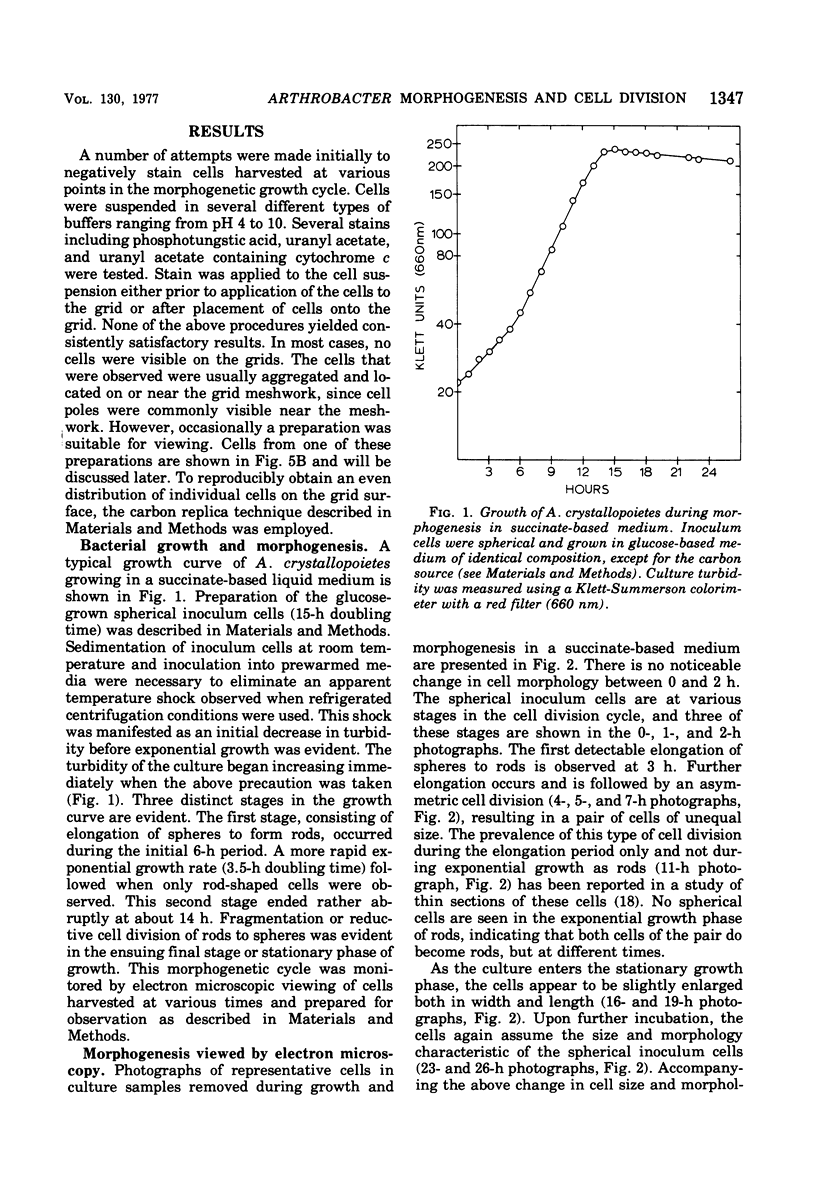
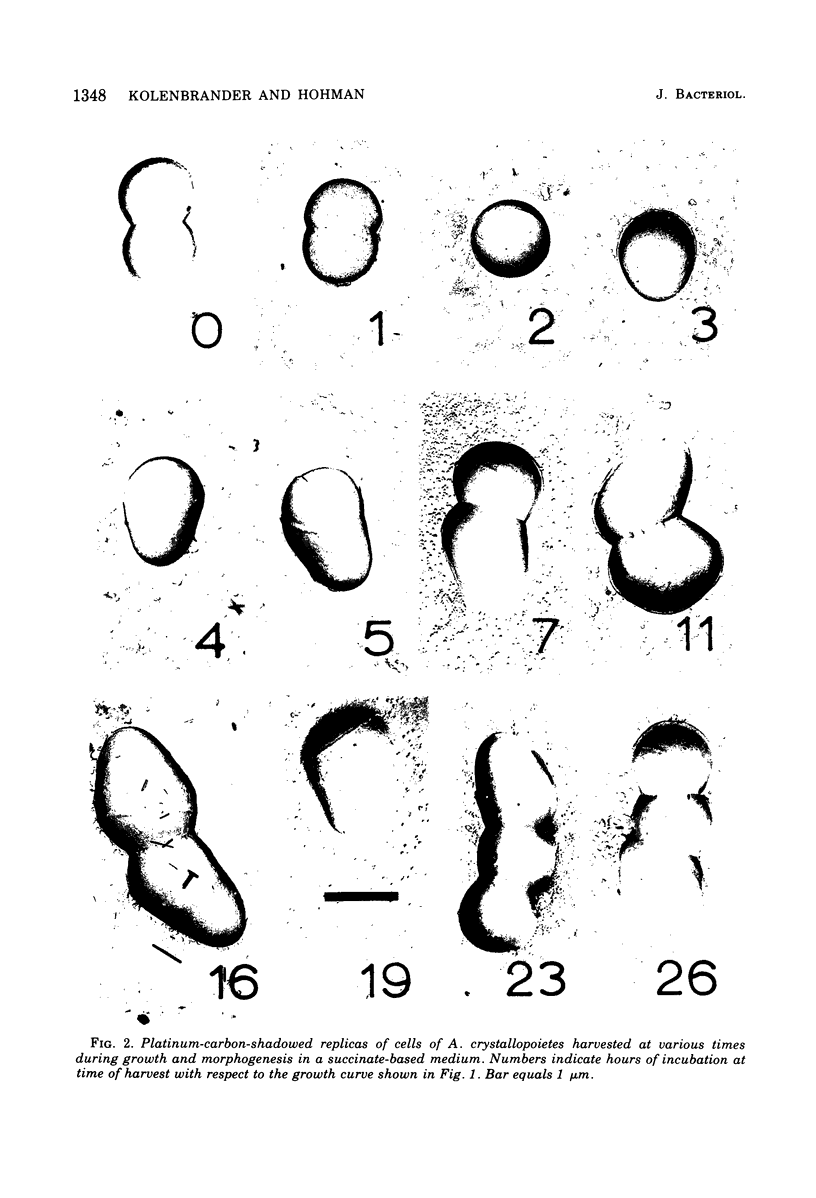
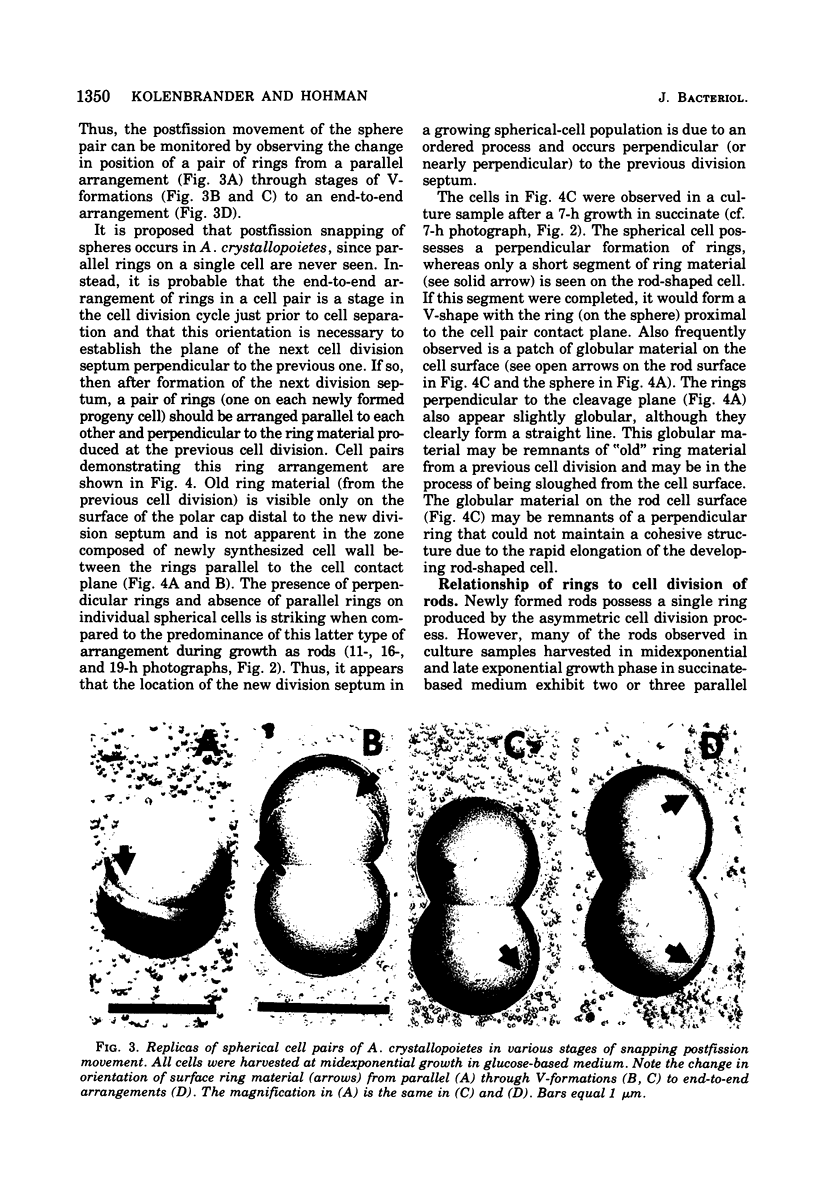
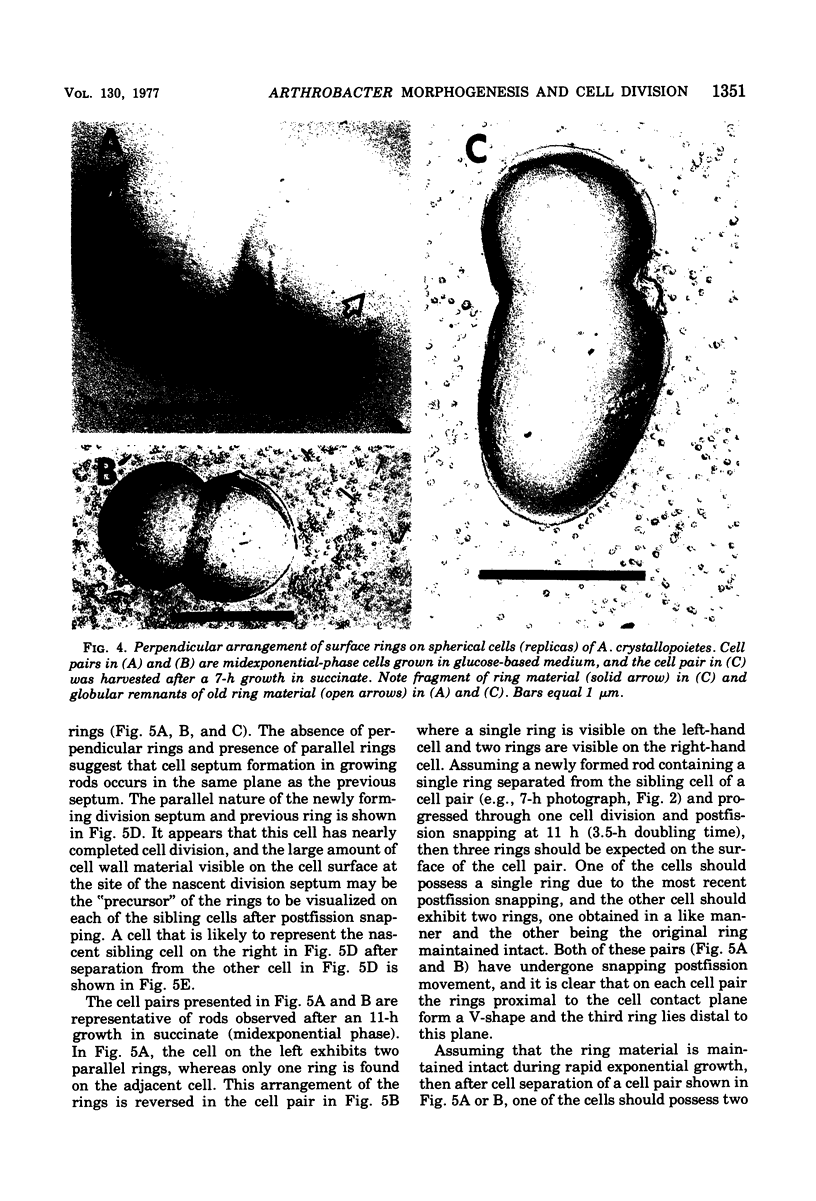
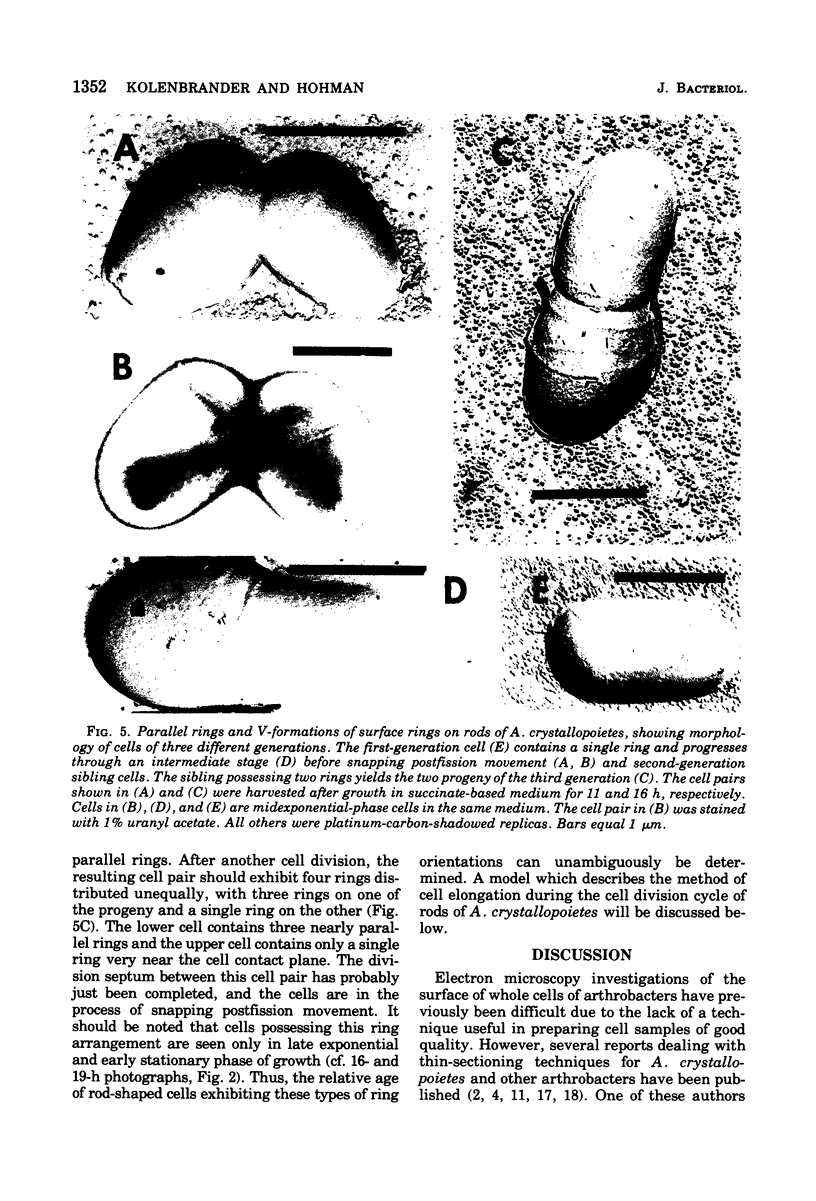
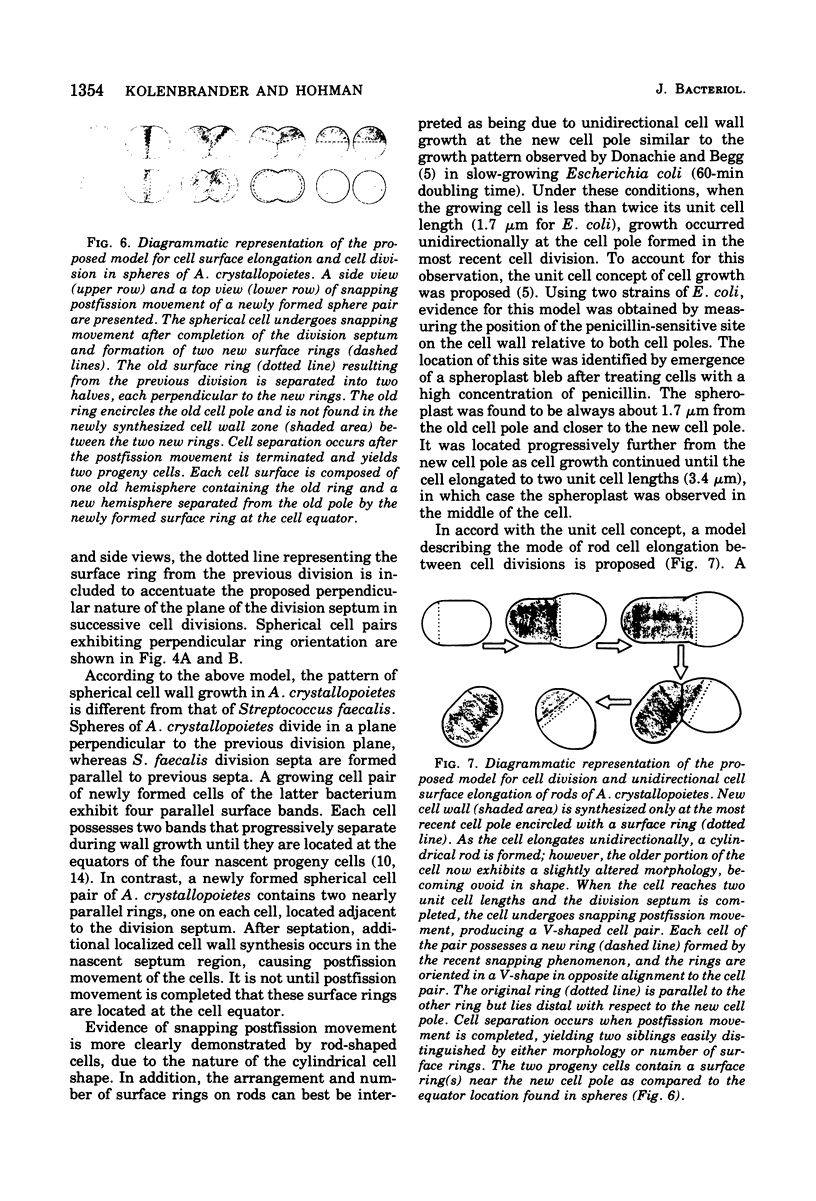
The whole cell ultrastructure during cell division and morphogenesis of Arthrobacter crystallopoietes was monitored using electron microscopic techniques. Glucose-grown spherical cells were inoculated into succinate-based medium. In this medium, the organism undergoes a morphogenetic cycle consisting of elongation of spheres to rods, exponential growth as rods, and fragmentation of rods to spherical cells. Raised bands or rings that encircled the cells were evident on the cell surface of both sphere- and rod-shaped cells. Many rod-shaped cells possessed two or more rings arranged adjacent to each other in a parallel orientation. At each cell division a new ring was formed on both siblings. However, as predicted by the proposed model of unidirectional cell growth and by maintaining a ring from the previous generation, unequal numbers of rings were observed on sibling cells. Only one ring was visible on most of the spherical inoculum cells, but in some cases a second ring perpendicular to the other ring was observed. Parallel rings were found on spherical cells resulting from fragmentation or reductive cell division of rods during the stationary growth phase. Thus, these spheres could be distinguished from inoculum spheres containing a single ring or perpendicular orientation of rings. The number of rings per cell and arrangement of rings on the cell surface of sibling cells after cell division, but before cell separation, are discussed with respect to cell age, cell division, and sphere-rod-sphere morphogenesis of A. crystallopoietes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H., Sigarlakie E., Faure J. C. Scanning and transmission electron microscopy of three strains of Bifidobacterium. Can J Microbiol. 1975 Sep;21(9):1305–1316. doi: 10.1139/m75-197. [DOI] [PubMed] [Google Scholar]
- Boylen C. W., Pate J. L. Fine structure of Arthrobacter crystallopoietes during long-term starvation of rod and spherical stage cells. Can J Microbiol. 1973 Jan;19(1):1–5. doi: 10.1139/m73-001. [DOI] [PubMed] [Google Scholar]
- Chan E. C., Gomersall M., Bernier J. The negative staining of "difficult" bacteria like Arthrobacter globiformis for electron microscopy. Can J Microbiol. 1974 Jun;20(6):901–903. doi: 10.1139/m74-139. [DOI] [PubMed] [Google Scholar]
- Chan E. C., Zyk B., Gomersall M. Biotin deficiency in Arthrobactger globiformis: comparative cell ultrastructure and nonreplacement of the vitamin by structurally unrelated compounds. J Bacteriol. 1973 Jan;113(1):394–402. doi: 10.1128/jb.113.1.394-402.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Farshtchi D., McClung N. M. Fine structure of Nocardia asteroides grown in a chemically defined medium. J Bacteriol. 1967 Jul;94(1):255–257. doi: 10.1128/jb.94.1.255-257.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces violaceoruber (S. coelicolor). III. The walls of the mycelium and spores. J Biophys Biochem Cytol. 1961 Aug;10:505–516. doi: 10.1083/jcb.10.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbo C. M., Beaton C. D., Coles N. W. Electron microscopy of the lysis of Staphylococcus aureus cell walls by Aeromonas lytic factor. J Bacteriol. 1967 Jun;93(6):1972–1975. doi: 10.1128/jb.93.6.1972-1975.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Pate J. L. Ultrastructural explanation for snapping postfission movements in Arthrobacter crystallopoietes. J Bacteriol. 1971 Jan;105(1):408–412. doi: 10.1128/jb.105.1.408-412.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- RANCOURT M. W., LECHEVALIER H. A. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF SPINY CONIDIA IN SPECIES OF STREPTOMYCES. Can J Microbiol. 1964 Jun;10:311–316. doi: 10.1139/m64-042. [DOI] [PubMed] [Google Scholar]
- STARR M. P., KUHN D. A. On the origin of V-forms in Arthrobacter atrocyaneus. Arch Mikrobiol. 1962;42:289–298. doi: 10.1007/BF00422046. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Martin J. T. Autolytic enzyme system of Streptococcus faecalis. IV. Electron microscopic observations of autolysin and lysozyme action. J Bacteriol. 1968 Nov;96(5):1803–1810. doi: 10.1128/jb.96.5.1803-1810.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L. The fine structure of Arthrobacter pascens and the development of mesosomes during the growth cycle. Can J Microbiol. 1968 Oct;14(10):1029–1034. doi: 10.1139/m68-173. [DOI] [PubMed] [Google Scholar]
- Ward C. M., Jr, Claus G. W. Gram characteristics and wall ultrastructure of Arthrobacter crystallopoietes during coccus-rod morphogenesis. J Bacteriol. 1973 Apr;114(1):378–389. doi: 10.1128/jb.114.1.378-389.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth H., Hopwood D. A. Septation during sporulation in Streptomyces coelicolor. J Gen Microbiol. 1970 Jan;60(1):51–59. doi: 10.1099/00221287-60-1-51. [DOI] [PubMed] [Google Scholar]