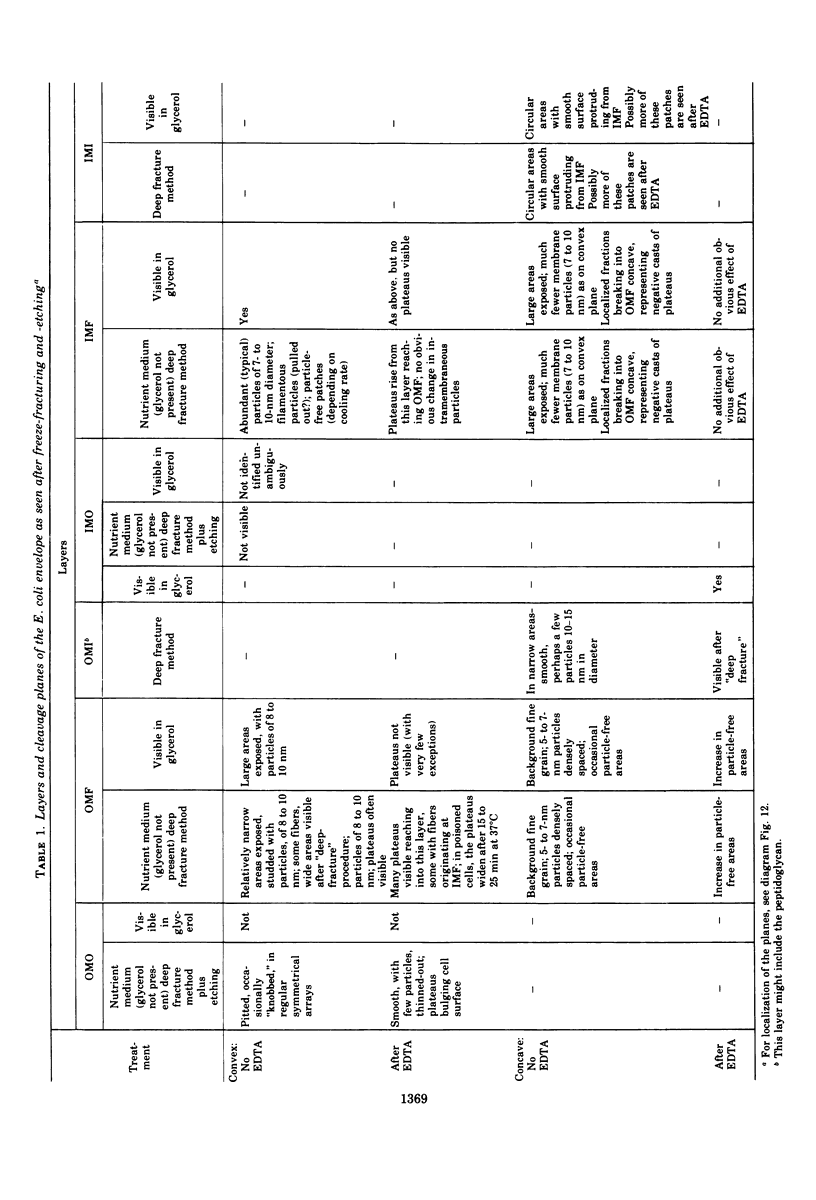
Abstract
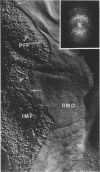
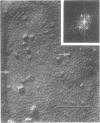
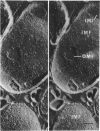
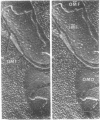
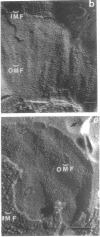
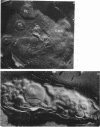
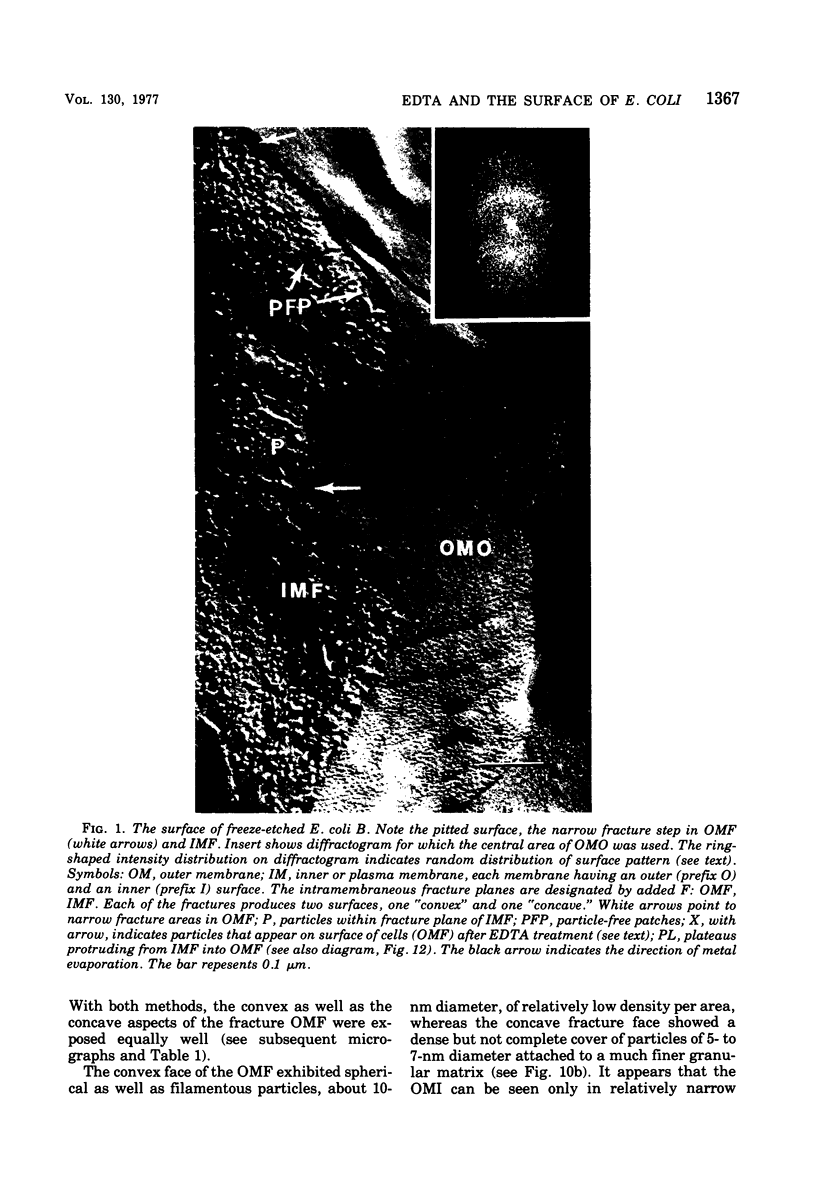
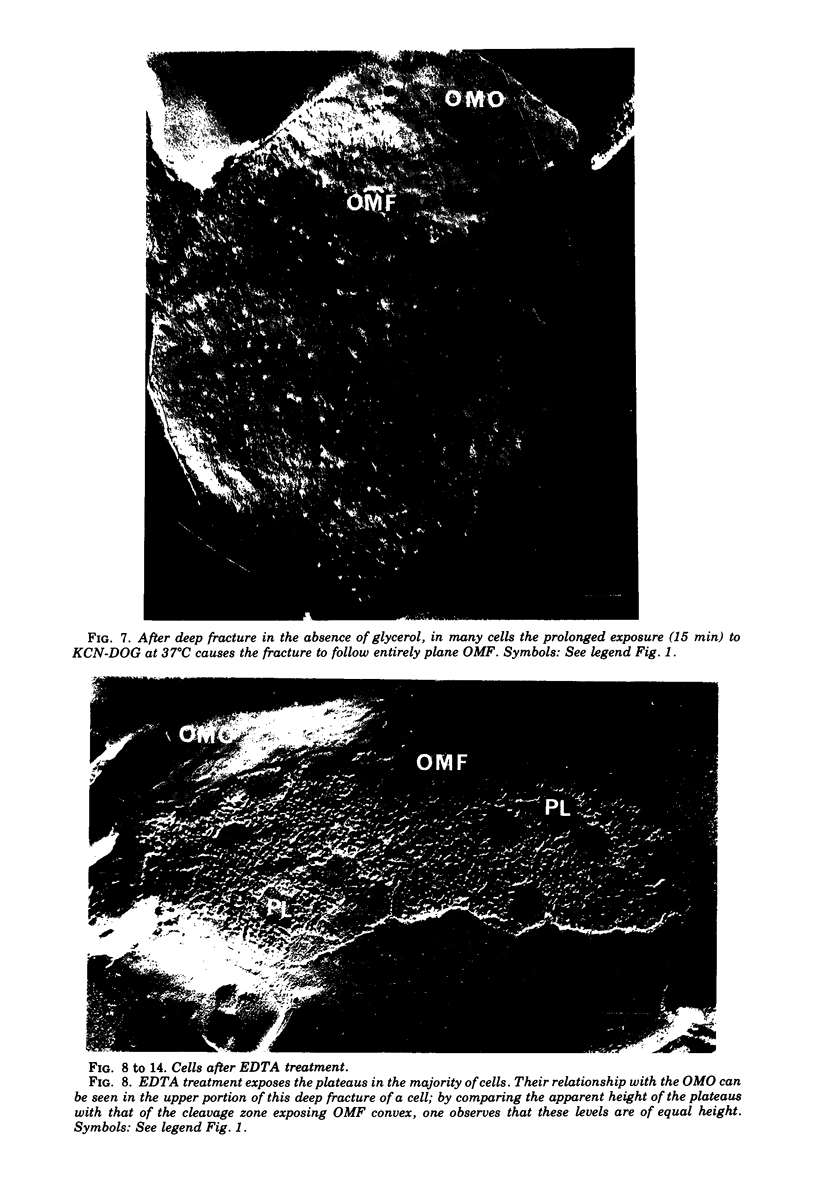
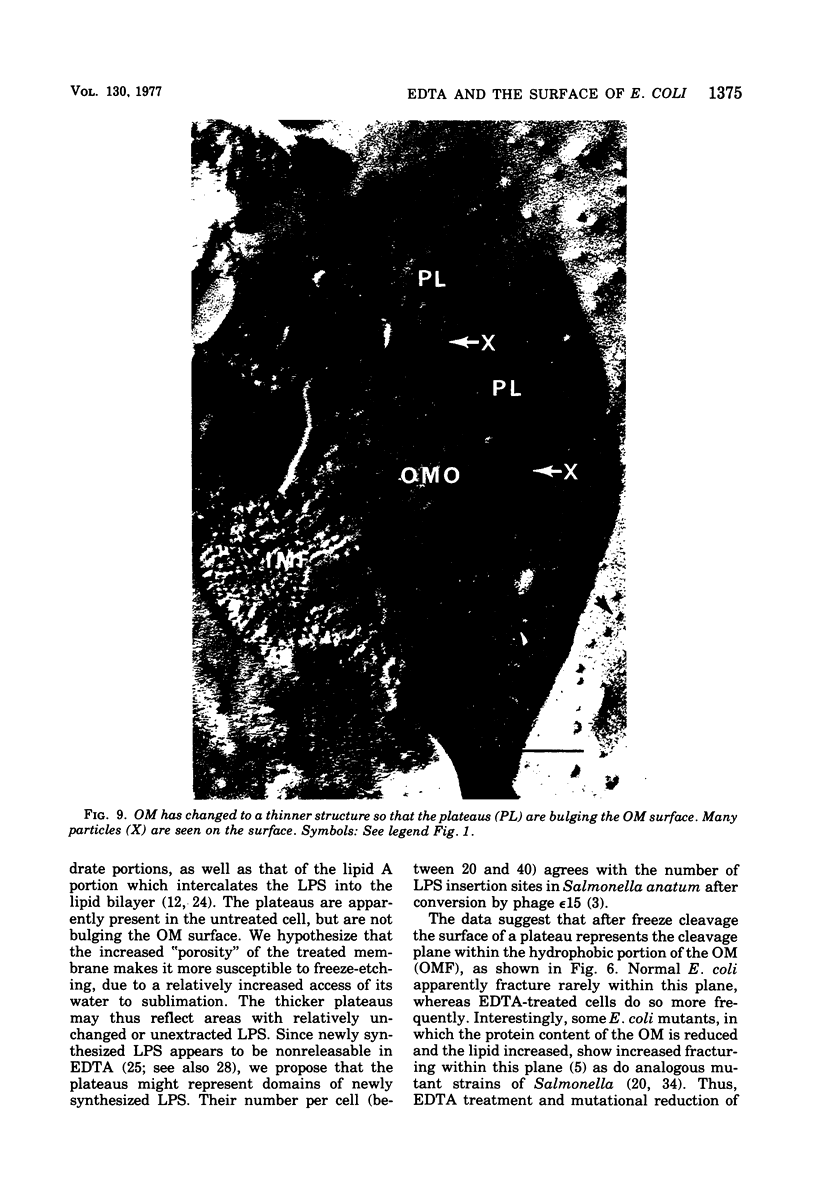
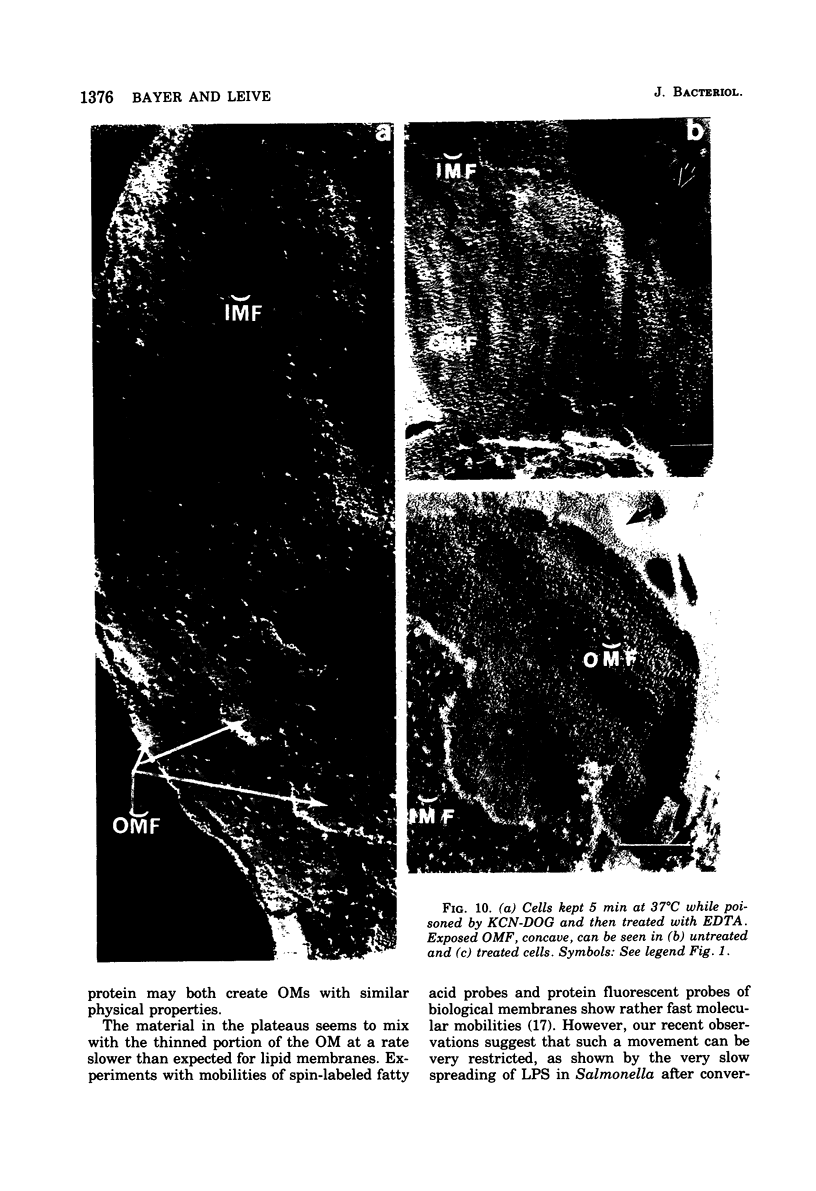
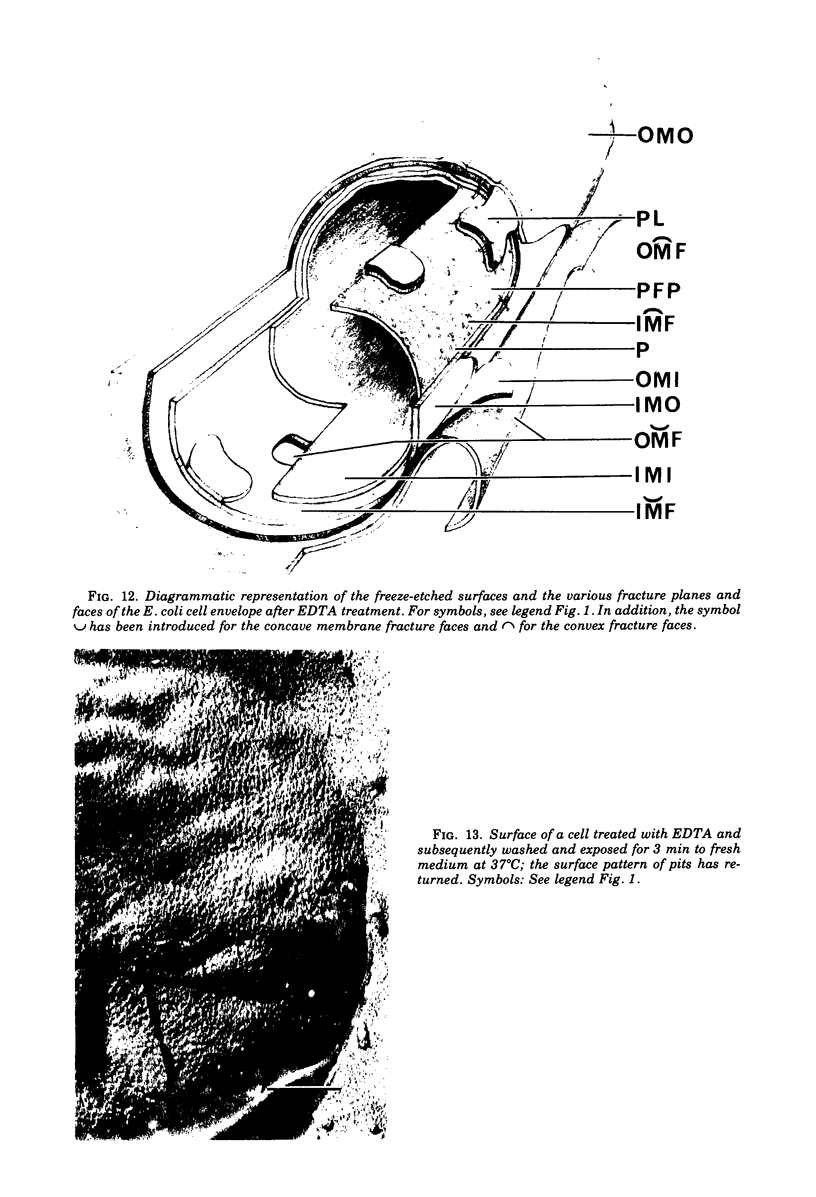
The effect of ethylenediaminetetraacetate (EDTA) on the envelope of two strains of Escherichia coli (B and Cla) was studied with freeze-fracturing methods. Untreated cells showed the outer membrane's outer surface with a fine texture of randomly spaced depressions of about 4.5-nm diameter; small areas with symmetrical arrangements of structural surface elements were also observed. The outer membrane's fracture plane revealed a random distribution of particles on its “concave” plane, only occasionally interrupted by particle-free areas. The “convex” aspect of the outer membrane's fracture plane showed only a few scattered particles. The cleavage plane of the inner membrane was often interrupted by many localized elevated plateaus, at which the cleaving process had, for short distances, switched to the outer membrane. The effects of EDTA treatment were mainly seen in the structure of the freeze-etched outer membrane: (i) the pits as well as the symmetrical surface elements of the outer membrane's outer surface had disappeared; (ii) a number of plateaus (about 20 to 50/cell) were seen at which a cleavage plane within the inner membrane had switched to the hydrophobic portion of the outer membrane (outer membrane's fracture plane). These plateaus were also visible in untreated cells; however, EDTA treatment apparently caused an increased exposure of plateaus. Surface areas, exposed by freeze-etching, revealed the underlying plateaus as elevations in the surface contour of the cell, suggesting a slower etching rate in the zones of the plateaus relative to the rest of the outer membrane. Well-defined, particle-free patches in the outer membrane's fracture plane, concave, were more frequent and larger in size after EDTA treatment than in the controls. In the presence of glycerol, the cells often cleaved in the outer membrane's fracture plane, but isolated plateaus were rarely observed. After metabolic poisoning of cells for 15 to 25 min at 37°C, the plateaus had widened. These data suggest that the material of the plateaus has a slow rate of lateral diffusion. Placement of EDTA-treated cells in fresh medium at 37°C caused, after 3 to 5 min, the reoccurrence of the pitted surface structure. We propose that the plateaus represent localized zones, at which newly synthesized lipopolysaccharide has been inserted.
Full text
PDF




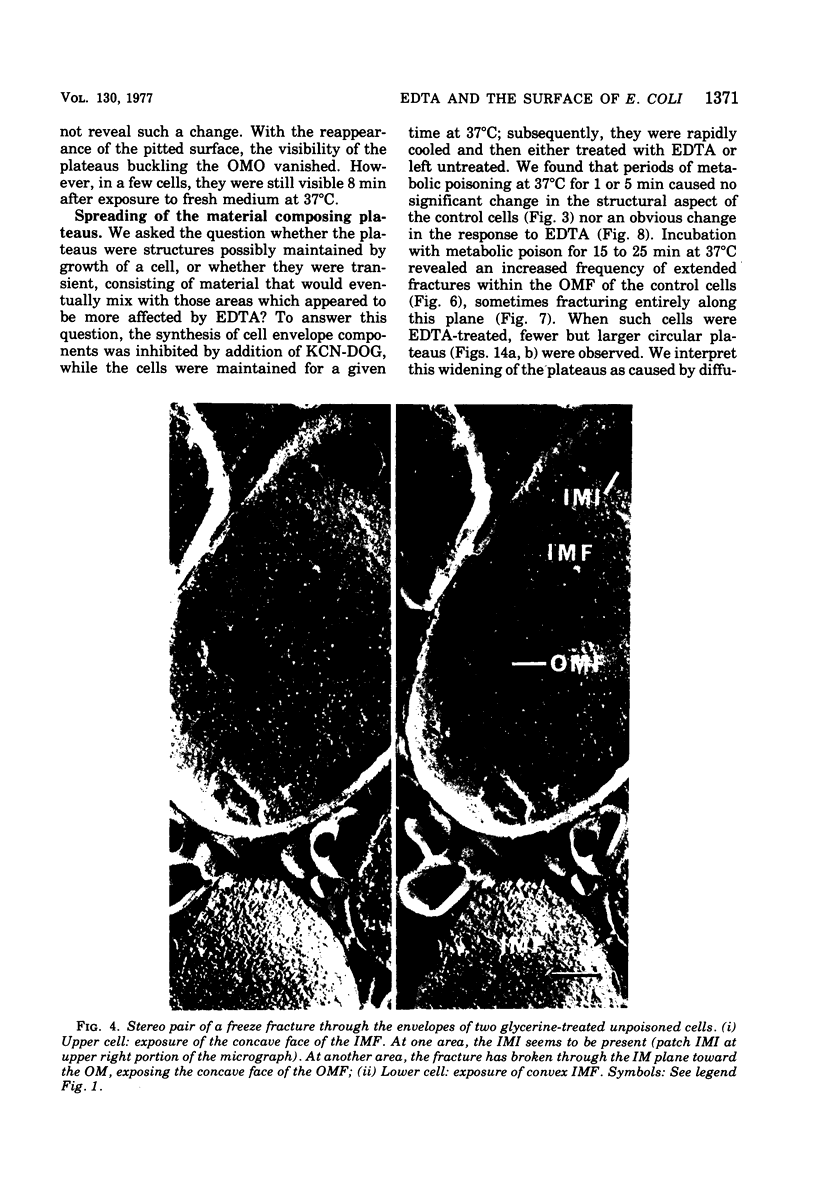
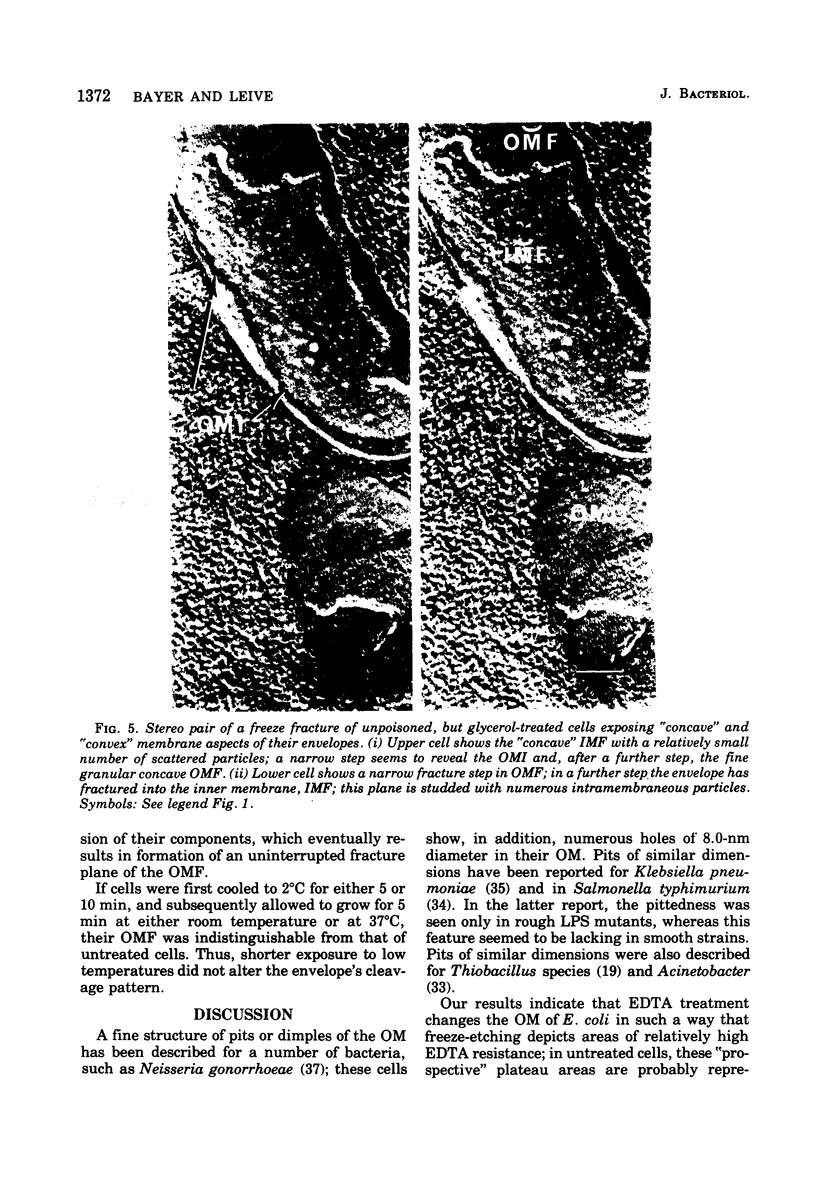
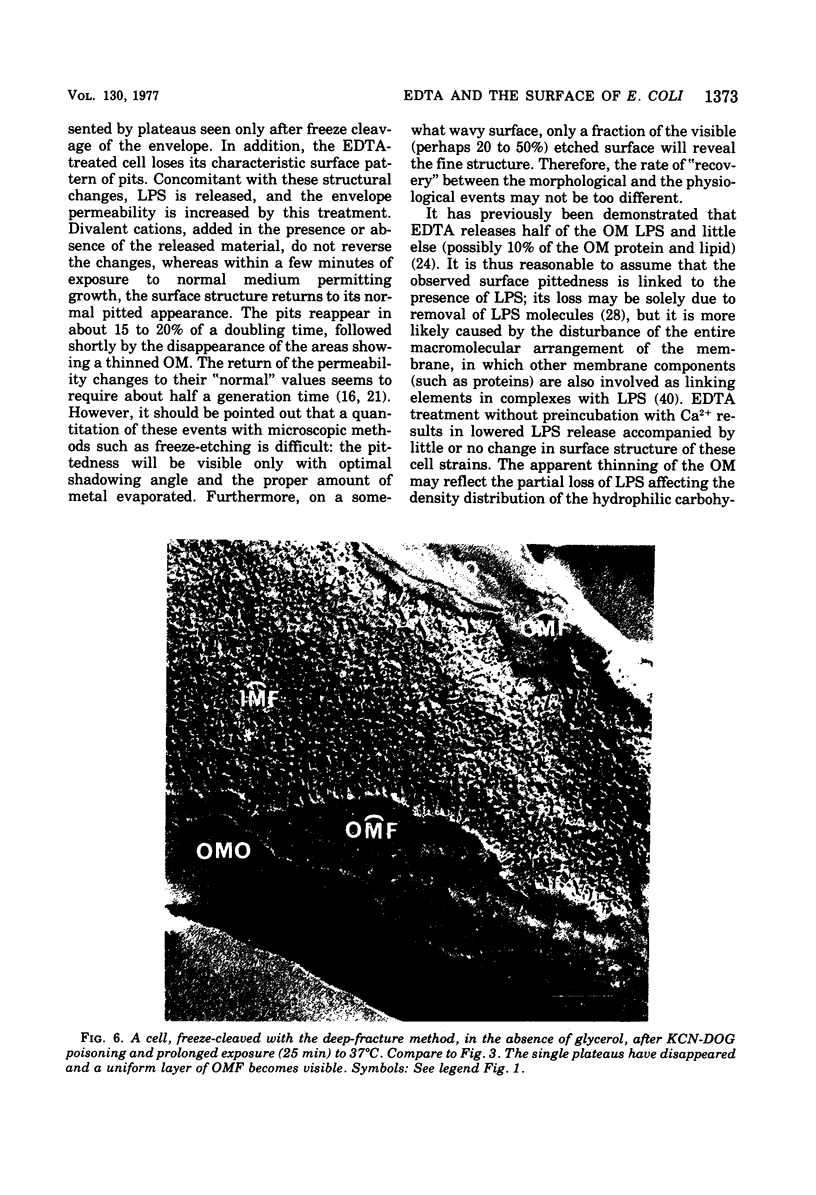



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Dolack M., Houser E. Effects of lipid phase transition of the freeze-cleaved envelope of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1563–1573. doi: 10.1128/jb.129.3.1563-1573.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Koplow J., Goldfine H. Alterations in envelope structure of heptose-deficient mutants of Escherichia coli as revealed by freeze-etching. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5145–5149. doi: 10.1073/pnas.72.12.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Ultrastructure and organization of the bacterial envelope. Ann N Y Acad Sci. 1974 May 10;235(0):6–28. doi: 10.1111/j.1749-6632.1974.tb43254.x. [DOI] [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Estimation of 3-deoxy sugars by means of the malonaldehyde-thiobarbituric acid reaction. Nature. 1960 Apr 9;186:155–156. doi: 10.1038/186155a0. [DOI] [PubMed] [Google Scholar]
- DUBOIS M., GILLES K., HAMILTON J. K., REBERS P. A., SMITH F. A colorimetric method for the determination of sugars. Nature. 1951 Jul 28;168(4265):167–167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A., Costerton J. W., Kerr K. A. Freeze-etching and x-ray diffraction of the isolated double-track layer from the cell wall of a gram-negative marine pseudomonad. J Bacteriol. 1973 Jan;113(1):445–451. doi: 10.1128/jb.113.1.445-451.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Ultrastructural study of polymyxin-resistant isolates of Pseudomonas aeruginosa. J Bacteriol. 1976 Jan;125(1):267–281. doi: 10.1128/jb.125.1.267-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Roth I. L., Eagon R. G. Freeze-etch study of Pseudomonas aeruginosa: localization within the cell wall of an ethylenediaminetetraacetate-extractable. J Bacteriol. 1973 Jan;113(1):417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulik-Krzywicki T. Structural studies of the associations between biological membrane components. Biochim Biophys Acta. 1975 Mar 25;415(1):1–28. doi: 10.1016/0304-4157(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., Chatterjee A. K., Sanderson K. E., Costerton J. W. Comparison of the cell envelope structure of a lipopolysaccharide-defective (heptose-deficient) strain and a smooth strain of Salmonella typhimurium. J Bacteriol. 1975 Nov;124(2):930–941. doi: 10.1128/jb.124.2.930-941.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Leive L. An equilibrium between two fractions of lipopolysaccharide in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1435–1439. doi: 10.1073/pnas.61.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 4. Typisierung von S. minnesota-R-Mutanten mittels Antibiotica. Zentralbl Bakteriol Orig. 1970 Apr;213(3):356–380. [PubMed] [Google Scholar]
- Sleytr U. B., Thornley M. J., Glauert A. M. Location of the fracture faces within the cell envelope of Acinetobacter species strain MJT-F5-5. J Bacteriol. 1974 May;118(2):693–707. doi: 10.1128/jb.118.2.693-707.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. Ultrastructure of the capsule of Klebsiella pneumoniae and slime of Enterobacter aerogenes revealed by freeze etching. Arch Mikrobiol. 1973 Nov 19;93(4):277–286. doi: 10.1007/BF00427925. [DOI] [PubMed] [Google Scholar]
- Stinnett J. D., Eagon R. G. A model system for studying protein-lipopolysaccharide synthesis, assembly, and insertion in the outer membrane of Pseudomonas aeruginosa. Can J Microbiol. 1975 Nov;21(11):1834–1841. doi: 10.1139/m75-266. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonocci. J Exp Med. 1972 Nov 1;136(5):1258–1271. doi: 10.1084/jem.136.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Higgins M. L. Effect of temperature on the distribution of membrane particles in Streptococcus faecalis as seen by the freeze-fracture technique. J Bacteriol. 1974 May;118(2):725–734. doi: 10.1128/jb.118.2.725-734.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. C., Heath E. C. Isolation and characterization of lipopolysaccharide protein from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2572–2576. doi: 10.1073/pnas.70.9.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool A. P., Nanninga N. Fracture faces in the cell envelope of Escherichia coli. J Bacteriol. 1971 Oct;108(1):474–481. doi: 10.1128/jb.108.1.474-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerikhuizen H., Kwak E., van Bruggen E. F., Witholt B. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim Biophys Acta. 1975 Dec 1;413(2):177–191. doi: 10.1016/0005-2736(75)90102-9. [DOI] [PubMed] [Google Scholar]