Abstract
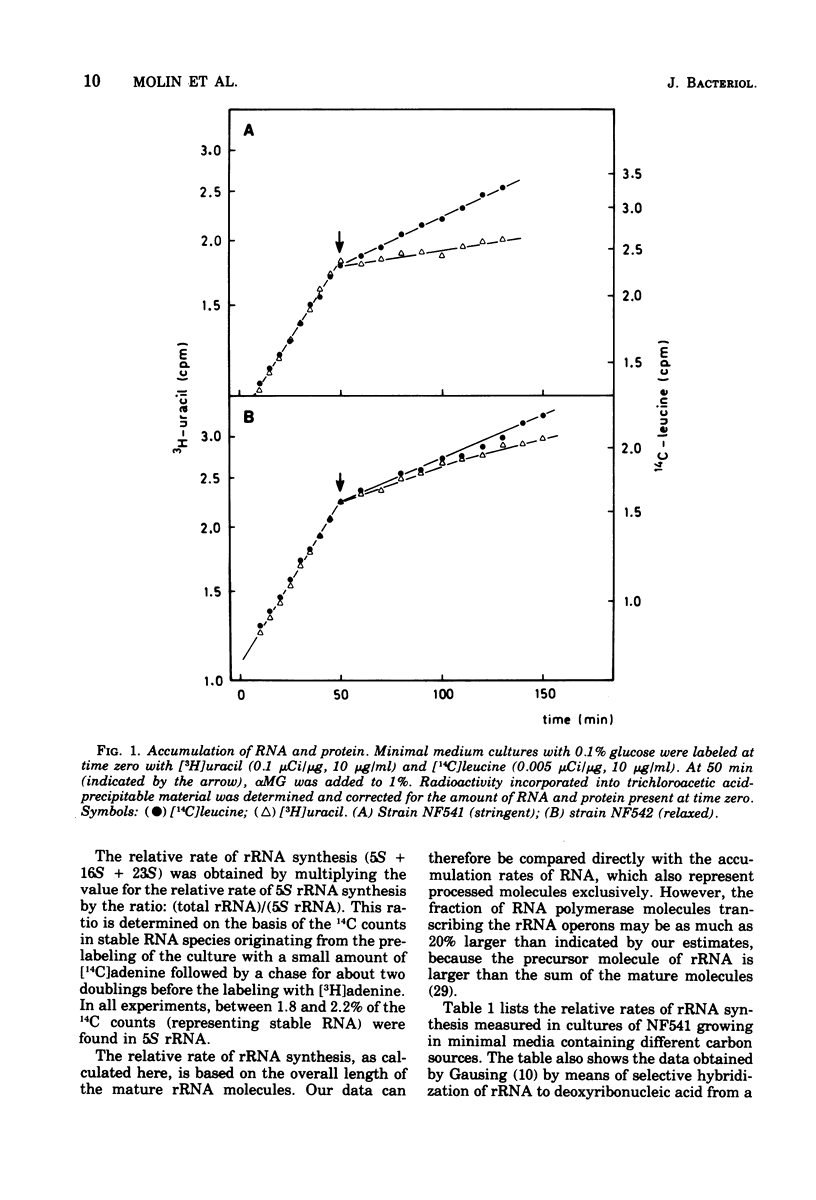
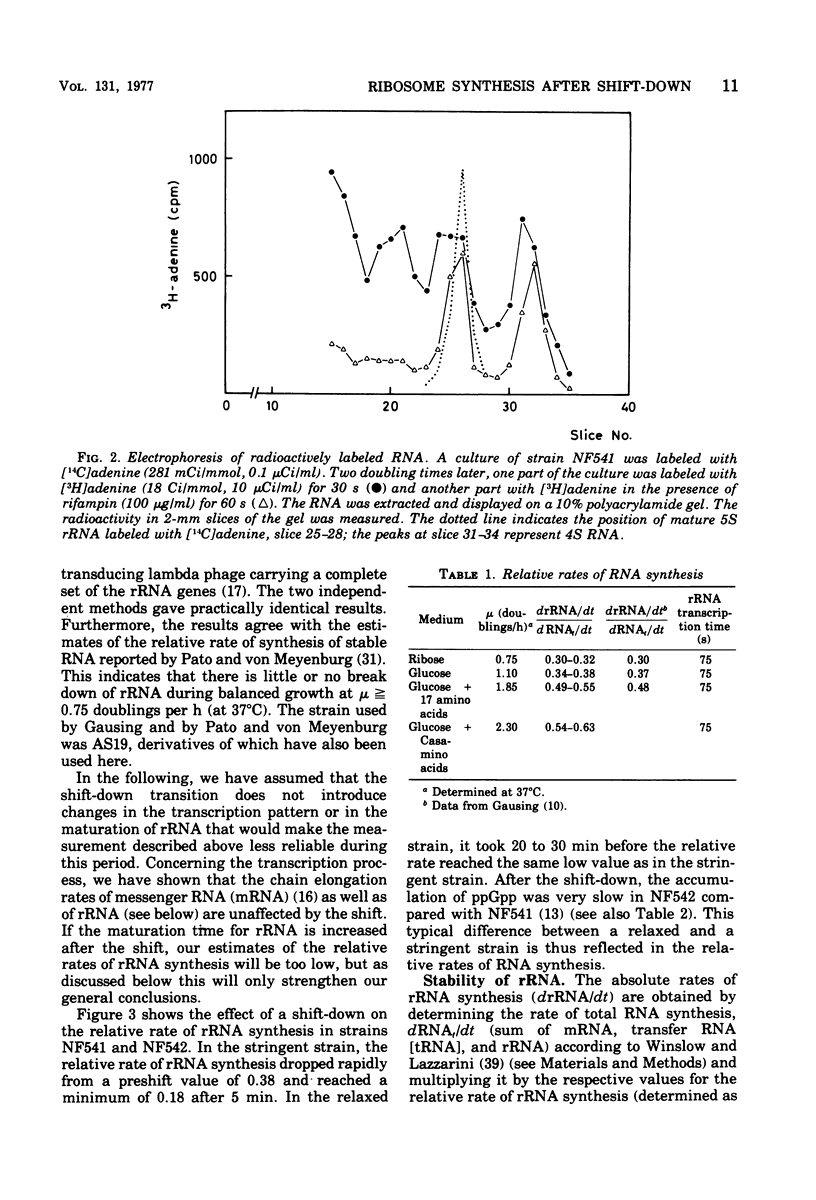
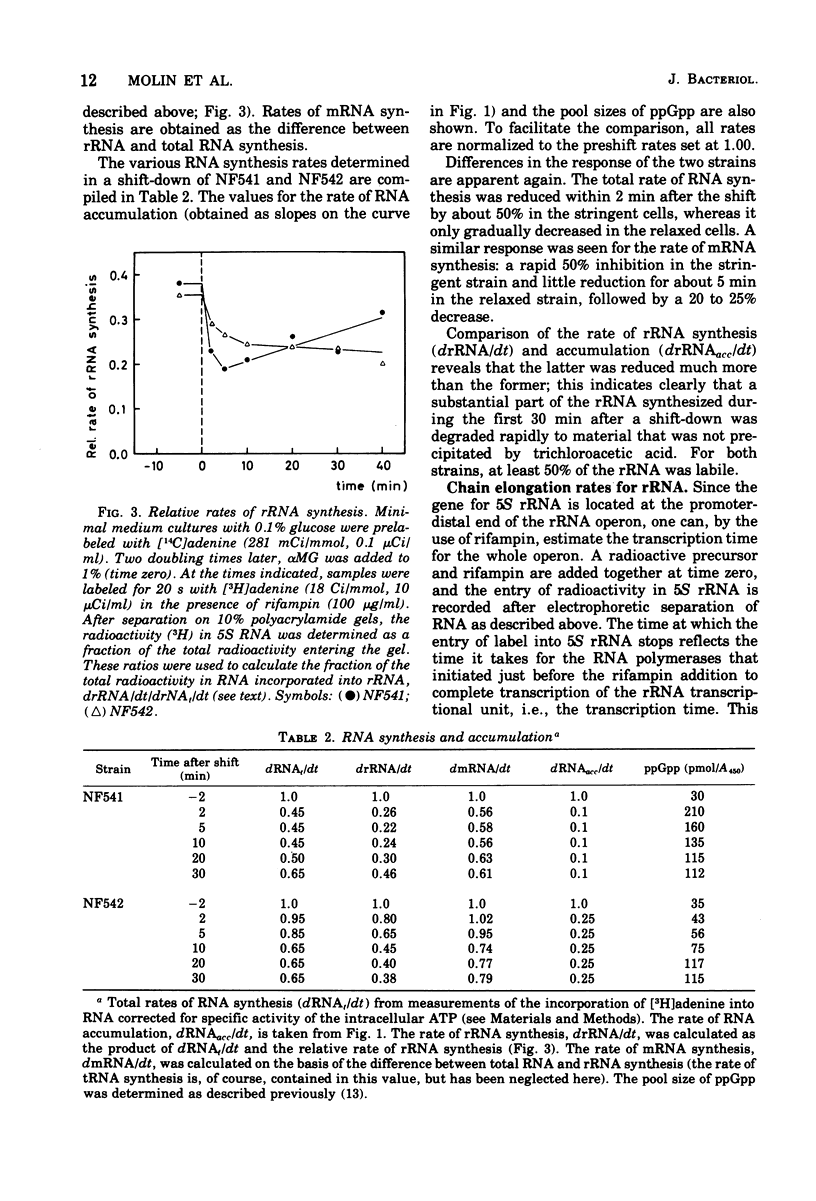
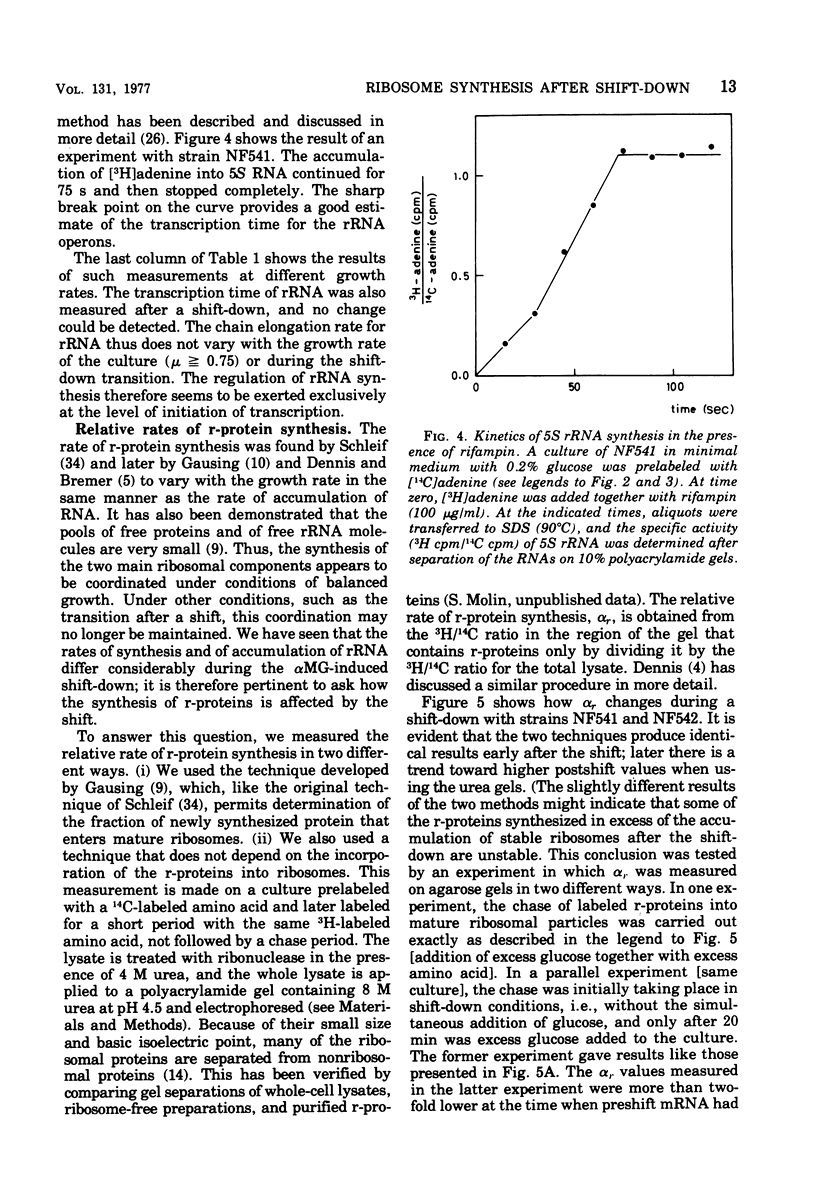
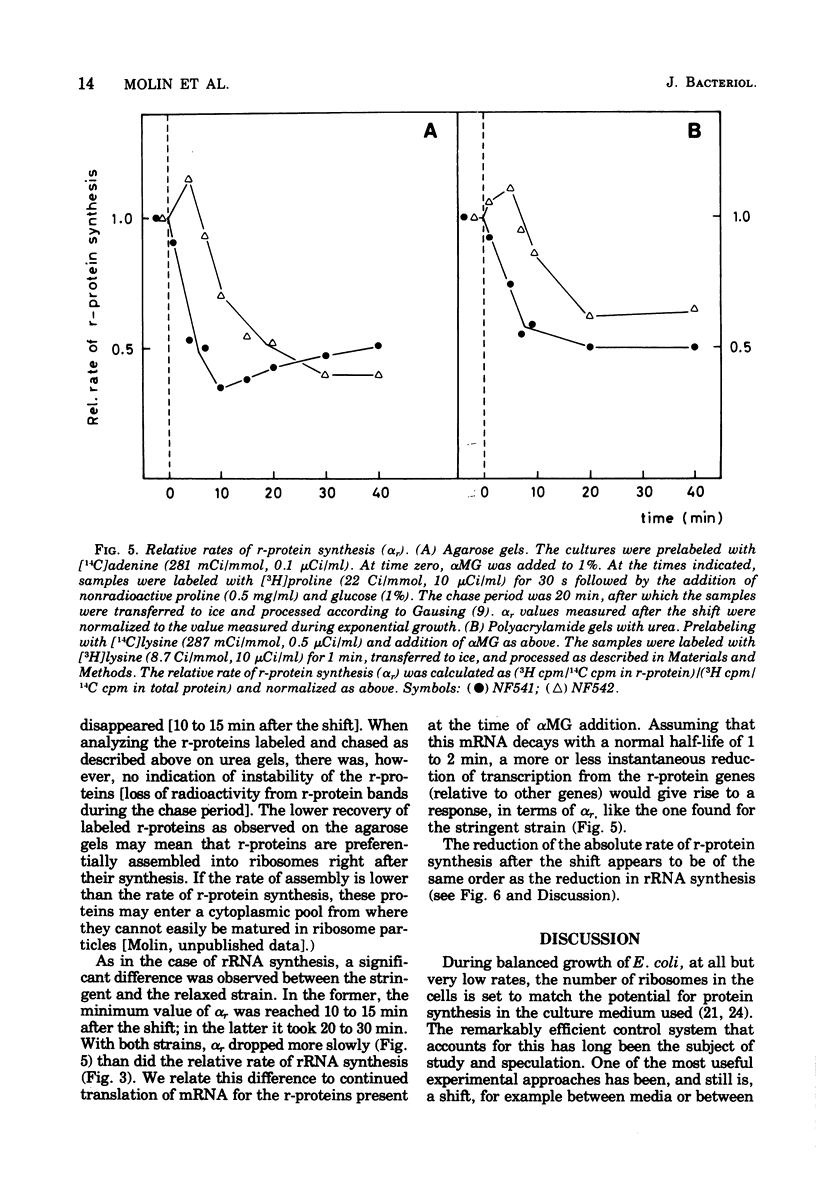
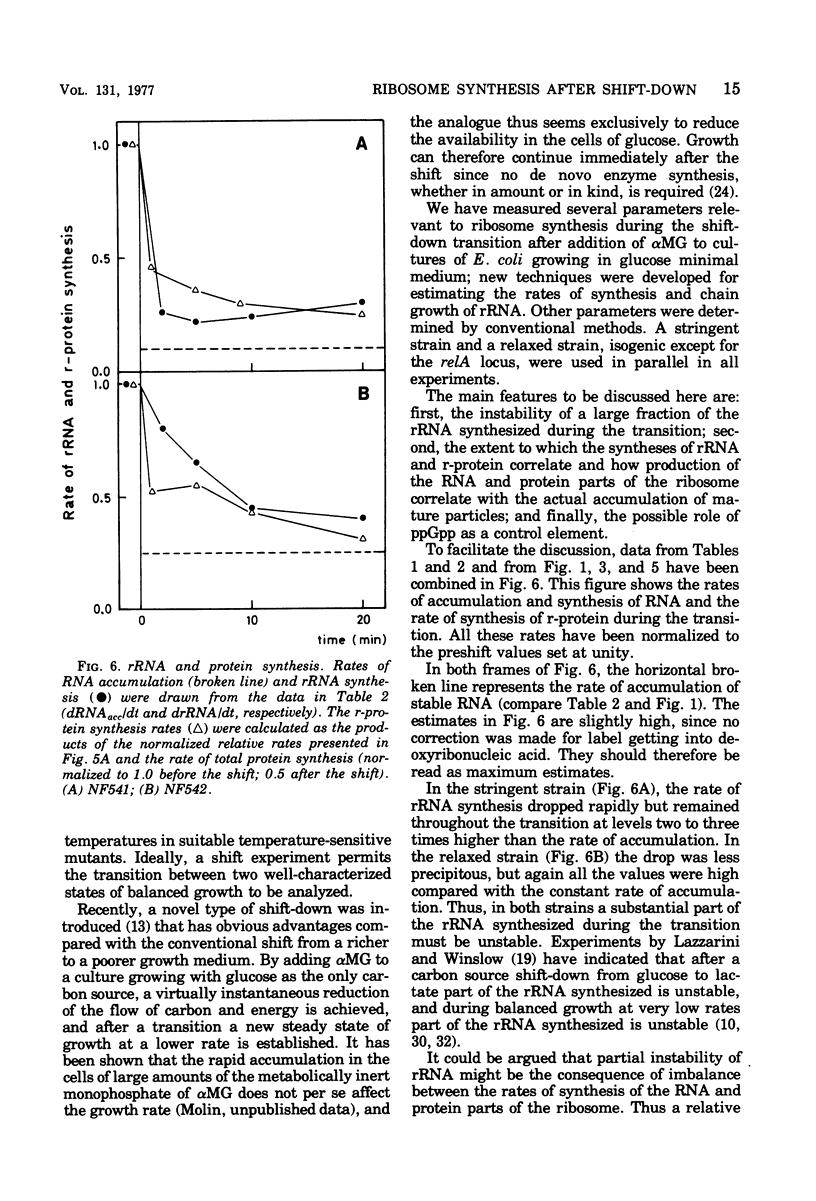
The rate of ribosome synthesis and accumulation in Escherichia coli during the transition after an energy source shift-down was analyzed. The shift was imposed on cultures of stringent and relaxed strains growing in glucose minimal medium by the addition of the glucose analogue α-methylglucoside. In the stringent strain, ribosome synthesis was almost instantaneously reduced after the shift, whereas the relaxed strain exhibited a more gradual response. The rate of messenger ribonucleic acid (mRNA) synthesis was affected similarly, though to a smaller extent. A comparison of the rates of synthesis and accumulation of ribosomal RNA (rRNA) and ribosomal proteins showed that far more ribosomal components were synthesized after the shift than were accumulated, indicating that a substantial part of the rRNA made after the shift was unstable. A new method was used to measure relative rates of rRNA synthesis and to estimate the transcription time for the rRNA operon under different conditions. In steady states of growth with growth rates ranging from 0.75 to 2.3 doublings/h, as well as during the transition after a shift-down, the transcription time of the rRNA operon was constant. The rate of synthesis of rRNA correlated during this transition – in contrast to the rate of accumulation (M. T. Hansen et al., J. Bacteriol. 122: 585-591, 1975) – with the ppGpp pool in the same way as has been observed during partial amino acid starvation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Differential rate of ribosomal protein synthesis in Escherichia coli B/r. J Mol Biol. 1974 Apr 15;84(3):407–422. doi: 10.1016/0022-2836(74)90449-5. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Regulation of the expression of ribosomal protein genes in Escherichia coli. J Mol Biol. 1975 Sep 5;97(1):61–76. doi: 10.1016/s0022-2836(75)80022-2. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. Synthesis of individual ribosomal proteins in Escherichia coli B/r. J Mol Biol. 1974 Oct 15;89(1):223–232. [PubMed] [Google Scholar]
- Fiil N. P., von Meyenburg K., Friesen J. D. Accumulation and turnover of guanosine tetraphosphate in Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):769–783. doi: 10.1016/s0022-2836(72)80037-8. [DOI] [PubMed] [Google Scholar]
- Gausing K. Ribosomal protein in E. coli: rate of synthesis and pool size at different growth rates. Mol Gen Genet. 1974 Mar 6;129(1):61–75. doi: 10.1007/BF00269266. [DOI] [PubMed] [Google Scholar]
- Gullov K. The size of transcriptional units for ribosomal proteins in Escherichia coli. Rates of synthesis of ribosomal proteins during a nuritional shift-up. Mol Gen Genet. 1974 May 31;130(3):271–274. doi: 10.1007/BF00268805. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Singh U. N. Biogenesis of ribosomes: free ribosomal protein pools in Escherichia coli. J Mol Biol. 1972 Aug 21;69(2):279–301. doi: 10.1016/0022-2836(72)90230-6. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Pato M. L., Molin S., Fill N. P., von Meyenburg K. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J Bacteriol. 1975 May;122(2):585–591. doi: 10.1128/jb.122.2.585-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Smith R. L., Gordon J. Ribosomal proteins: simplification of the methods to prepare them for gel electrophoresis. Anal Biochem. 1975 Jul;67(1):110–114. doi: 10.1016/0003-2697(75)90277-8. [DOI] [PubMed] [Google Scholar]
- Johnsen K., Molin S., Karlström O., Maaloe O. Control of protein synthesis in Escherichia coli: analysis of an energy source shift-down. J Bacteriol. 1977 Jul;131(1):18–29. doi: 10.1128/jb.131.1.18-29.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Forchhammer J. Evidence for reduced breakdown of messenger RNA during blocked transcription or translation in Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):593–606. doi: 10.1016/0022-2836(69)90361-1. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. Synthesis of a large precursor to ribosomal RNA in a mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3361–3365. doi: 10.1073/pnas.70.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris T. E., Koch A. L. Effect of growth rate on the relative rates of synthesis of messenger, ribosomal and transfer RNA in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):633–649. doi: 10.1016/0022-2836(72)90088-5. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Winslow R. M. A consequence of the rel gene during a glucose to lactate downshift in Escherichia coli. The rates of ribonucleic acid synthesis. J Biol Chem. 1971 Aug 10;246(15):4872–4877. [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. The rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Mar 10;244(5):1128–1136. [PubMed] [Google Scholar]



