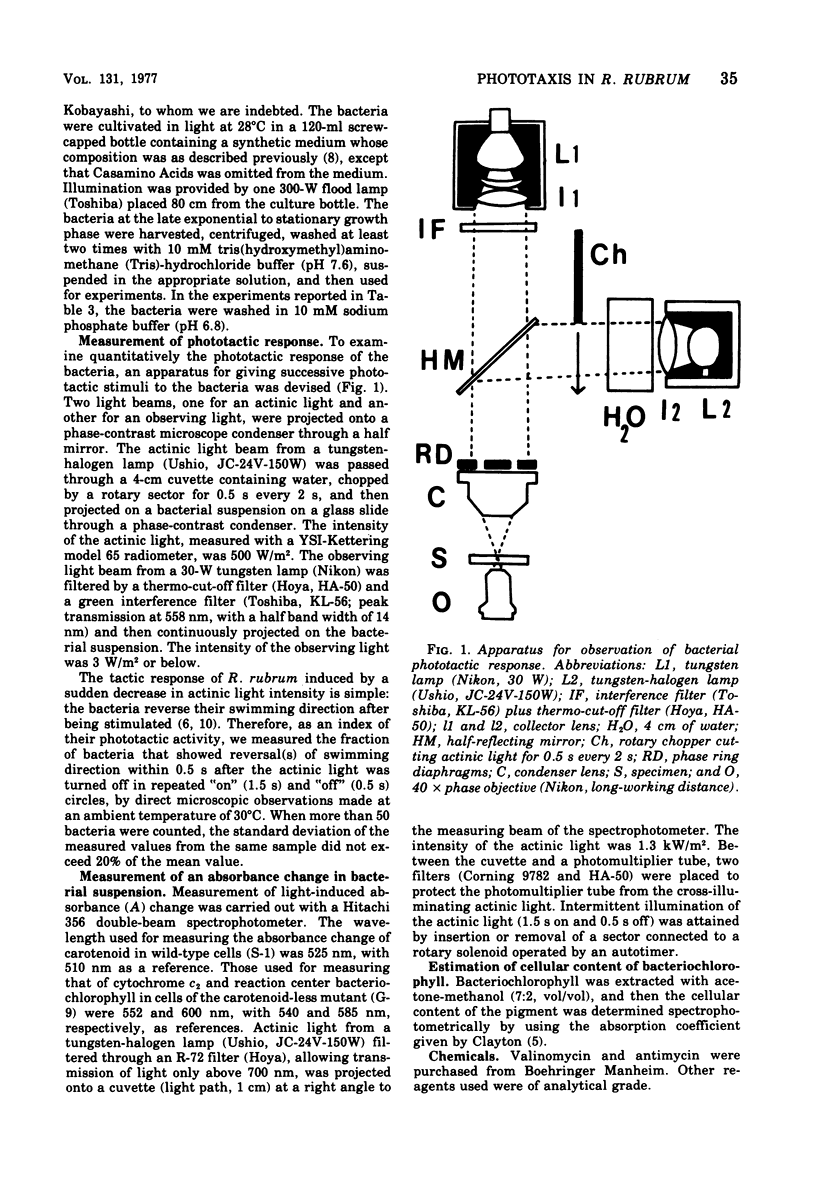
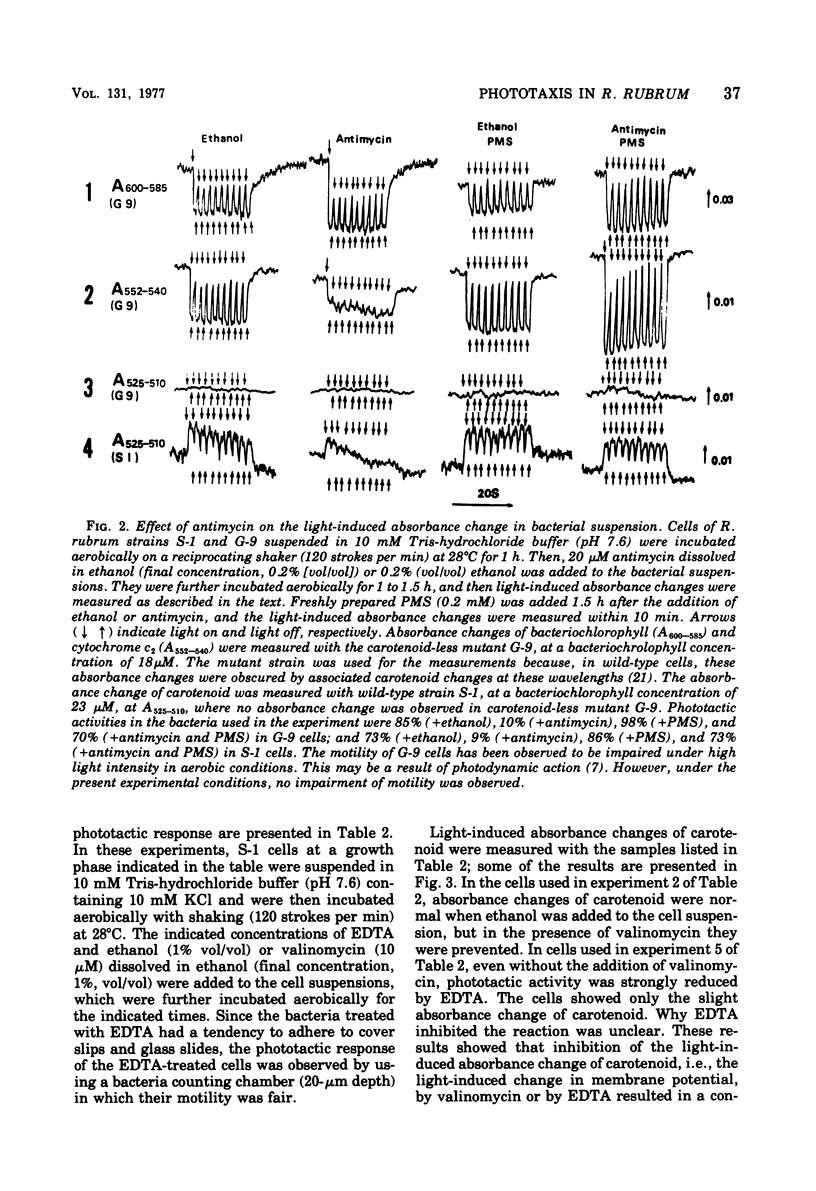
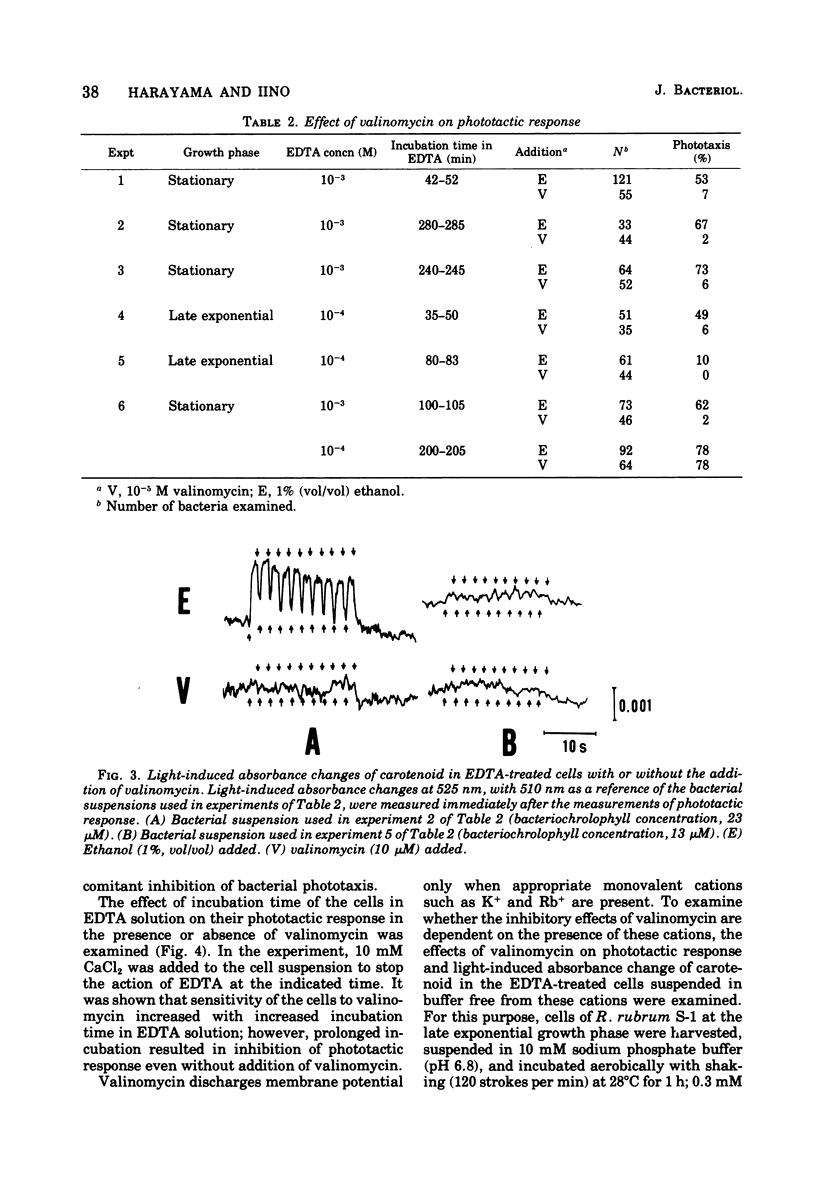
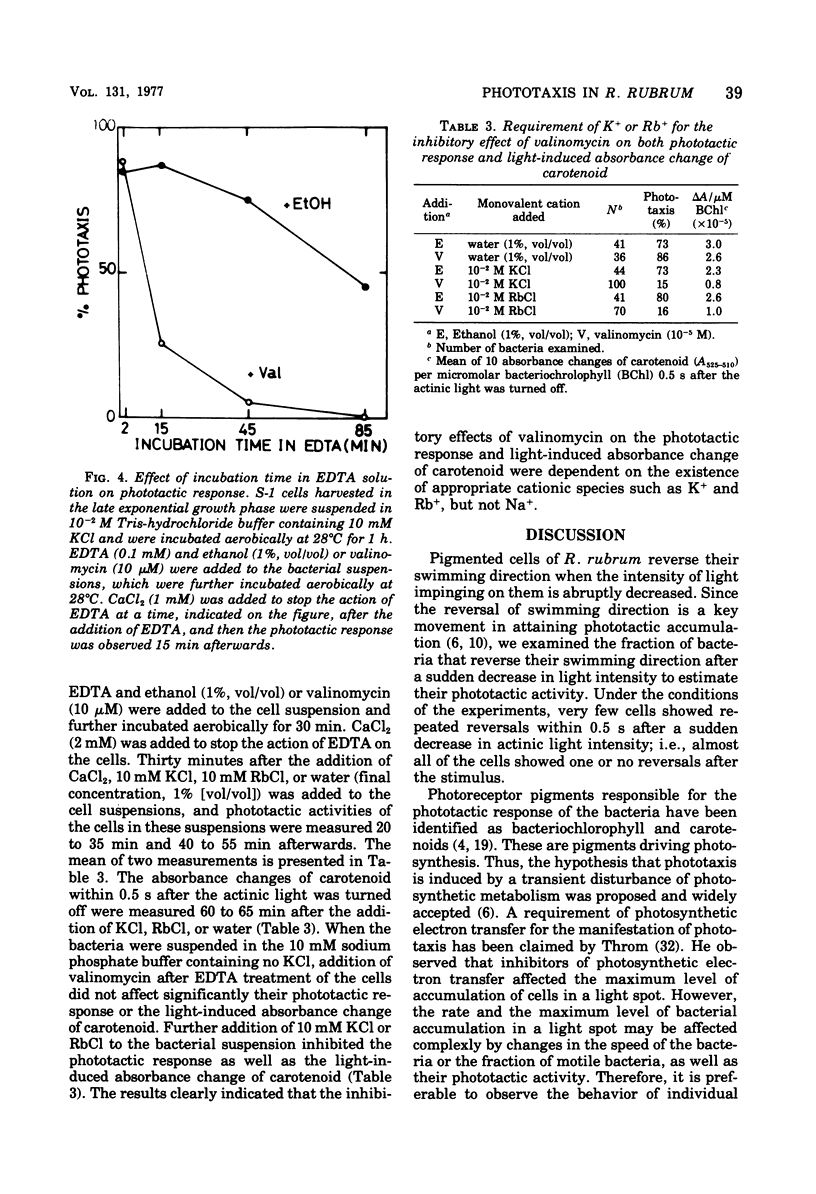
Abstract
Cells of the photosynthetic bacterium Rhodospirillum rubrum cultivated anaerobically in light show phototaxis. The behavior of individual cells in response to the phenomenon is reversal(s) of the swimming direction when the intensity of the light available to them abruptly decreases. The tactic response was inhibited by antimycin, an inhibitor of the photosynthetic electron transfer system. The inhibitory effect of antimycin was overcome by phenazine methosulfate. Motility of the cells was not impaired by antimycin under aerobic conditions. Valinomycin plus potassium also inhibited their phototactic response; however, valinomycin or potassium alone had no effect. A change in membrane potential of the cells was measured as an absorbance change of carotenoid. Changes in the membrane potential caused by "on-off" light were prevented by antimycin and by valinomycin plus potassium, but not by antimycin plus phenazine methosulfate nor valinomycin or potassium alone. The results indicated that the phototactic response of R. rubrum is mediated by a sudden change in electron flow in the photosynthetic electron transfer system, and that the membrane potential plays an important role in manifestation of the response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Bacterial behaviour. Nature. 1975 Apr 3;254(5499):389–392. doi: 10.1038/254389a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Tedesco P. M. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. Studies in the phototaxis of Rhodospirillum rubrum. I. Action spectrum, growth in green light, and Weber law adherence. Arch Mikrobiol. 1953;19(2):107–124. doi: 10.1007/BF00446395. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M., SISTROM W. R., COHENBAZIRE G., STANIER R. Y., CALVIN M. Function of carotenoids in photosynthesis. Nature. 1955 Dec 24;176(4495):1211–1215. doi: 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- Harayama S., Iino T. Phototactic response of aerobically cultivated Rhodospirillum rubrum. J Gen Microbiol. 1976 May;94(1):173–179. doi: 10.1099/00221287-94-1-173. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt W. Phototaxis in plants. Int Rev Cytol. 1966;19:267–299. [PubMed] [Google Scholar]
- Hazelbauer G. L. Maltose chemoreceptor of Escherichia coli. J Bacteriol. 1975 Apr;122(1):206–214. doi: 10.1128/jb.122.1.206-214.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand E., Dencher N. Two photosystems controlling behavioural responses of Halobacterium halobium. Nature. 1975 Sep 4;257(5521):46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. The high energy state in chromatophores from Rhodopseudomonas spheroides. FEBS Lett. 1969 Aug;4(3):185–189. doi: 10.1016/0014-5793(69)80230-9. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Dutton P. L. The kinetic and redox potentiometric resolution of the carotenoid shifts in Rhodopseudomonas spheroides chromatophores: their relationship to electric field alterations in electron transport and energy coupling. Biochim Biophys Acta. 1973 Oct 19;325(1):102–113. doi: 10.1016/0005-2728(73)90155-2. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr Chemotaxis as a model for sensory systems. FEBS Lett. 1974 Mar 23;40(0):suppl–suppl:S9. doi: 10.1016/0014-5793(74)80683-6. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILATZ J. M., MANTEN A. The quantitative determination of the spectral distribution of phototactic sensitivity in the purple bacterium Rhodospirillum rubrum. Biochim Biophys Acta. 1953 May;11(1):17–27. doi: 10.1016/0006-3002(53)90004-3. [DOI] [PubMed] [Google Scholar]
- NISHIMURA M., CHANCE B. Studies on the electron-transfer systems in photosynthetic bacteria. I. The light-induced absorption-spectrum changes and the effect of phenylmercuric acetate. Biochim Biophys Acta. 1963 Jan 15;66:1–16. doi: 10.1016/0006-3002(63)91162-4. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Control of tumbling in bacterial chemotaxis by divalent cation. J Bacteriol. 1976 May;126(2):706–711. doi: 10.1128/jb.126.2.706-711.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotactic repellents of Bacillus subtilis. J Mol Biol. 1976 Jan 5;100(1):103–108. doi: 10.1016/s0022-2836(76)80037-x. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science. 1975 Sep 5;189(4205):802–805. doi: 10.1126/science.808854. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Genetics of chemotactic behavior in bacteria. Cell. 1975 Mar;4(3):183–188. doi: 10.1016/0092-8674(75)90166-x. [DOI] [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Koshland D. E., Jr Quantitation of the sensory response in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Feb;72(2):710–713. doi: 10.1073/pnas.72.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grondelle R., Duysens L. N., van der Wal H. N. Function of three cytochromes in photosynthesis of whole cells of Rhodospirillum rubrum as studied by flash spectroscopy. Evidence for two types of reaction center. Biochim Biophys Acta. 1976 Nov 9;449(2):169–187. doi: 10.1016/0005-2728(76)90131-6. [DOI] [PubMed] [Google Scholar]



