Abstract
HIV-1 specifically incorporates the peptidyl prolyl isomerase cyclophilin A (CyPA), the cytosolic receptor for the immunosuppressant cyclosporin A (CsA). HIV-1 replication is inhibited by CsA as well as by nonimmunosuppressive CsA analogues that bind to CyPA and interfere with its virion association. In contrast, the related simian immunodeficiency virus SIVmac, which does not interact with CyPA, is resistant to these compounds. The incorporation of CyPA into HIV-1 virions is mediated by a specific interaction between the active site of the enzyme and the capsid (CA) domain of the HIV-1 Gag polyprotein. We report here that the transfer of HIV-1 CA residues 86–93, which form part of an exposed loop, to the corresponding position in SIVmac resulted in the efficient incorporation of CyPA and conferred an HIV-1-like sensitivity to a nonimmunosuppressive cyclosporin. HIV-1 CA residues 86–90 were also sufficient to transfer the ability to efficiently incorporate CyPA, provided that the length of the CyPA-binding loop was preserved. However, the resulting SIVmac mutant required the presence of cyclosporin for efficient virus replication. The results indicate that the presence or absence of a type II tight turn adjacent to the primary CyPA-binding site determines whether CyPA incorporation enhances or inhibits viral replication. By demonstrating that CyPA-binding-site residues can induce cyclosporin sensitivity in a heterologous context, this study provides direct in vivo evidence that the exposed loop between helices IV and V of HIV-1 CA not merely constitutes a docking site for CyPA but is a functional target of this cellular protein.
The host protein cyclophilin A (CyPA) is specifically incorporated into HIV-1 virions via an interaction with the capsid (CA) domain of the Gag polyprotein Pr55gag (1–5). CyPA is an abundant cytosolic peptidyl-prolyl cis–trans isomerase that is believed to assist in protein folding (6–9). CyPA has a high affinity for the immunosuppressant cyclosporin A (CsA), which binds to the active site of the enzyme and inhibits its rotamase activity (6–11). In contrast, a novel nuclease activity associated with purified CyPA is not inhibited by CsA (12). The complex of CsA with CyPA inhibits calcineurin, a serine/threonine phosphatase crucial for T cell activation (13). However, nonimmunosuppressive CsA analogues have been described that retain a high affinity for CyPA yet do not suppress T cell activation because the complex with CyPA does not bind to calcineurin (14–16).
CyPA is found in HIV-1 virions at levels similar to those of virally encoded enzymes but is not incorporated into other retroviruses (2–4). CsA and nonimmunosuppressive CsA analogues such as SDZ NIM 811 inhibit the incorporation of CyPA into HIV-1 virions (2, 3), HIV-1 virion infectivity (3, 15, 16), and HIV-1 replication (2, 3, 15–17). Although CsA and SDZ NIM 811 have no discernible effect on HIV-1 morphology, virions produced in the presence of these compounds are defective at an early step in the virus life cycle (18, 19). SDZ NIM 811 is inactive against SIVmac, a primate lentivirus related to HIV-1 that does not incorporate CyPA (3, 15). However, SIVmac packages CyPA and becomes sensitive to SDZ NIM 811 upon replacement of its CA domain, which forms the core of the virion, by that of HIV-1 (5). This observation and the finding that mutations in the HIV-1 CA domain can confer resistance to cyclosporins (20, 21) provide genetic evidence that HIV-1 CA is the functional target of CyPA.
The recently solved crystal structures of CyPA bound to the N-terminal domain of HIV-1 CA or to a 25-mer fragment of CA show that CA residues 86–93, which form part of a solvent-exposed loop (22, 23), bind in the active site of the rotamase (24, 25). The primary CyPA-binding site overlaps with a region of CA that is highly variable among different groups of primate lentiviruses (2). We report here that the transfer of an 8-aa segment that includes HIV-1 CA residues 86–93 to the corresponding position in SIVmac CA leads to the efficient incorporation of CyPA and confers an HIV-1-like sensitivity toward the nonimmunosuppressive cyclosporin SDZ NIM 811. The transfer of smaller segments that did not include residues that contribute to a type II tight turn in the HIV-1 CyPA-binding loop also led to the efficient incorporation of CyPA into SIVmac virions. However, the resulting chimeras exhibited a cyclosporin-dependent rather than a cyclosporin-sensitive phenotype. These results are consistent with a recently proposed model in which CyPA modulates CA–CA interactions that involve the type II tight turn adjacent to its primary binding site (24, 26).
MATERIALS AND METHODS
Plasmids.
The parental HIV-1 provirus used in this study, HXBH10, is a vpu+ variant of the infectious HXB2 molecular clone of HIV-1 (27). The infectious SIVmac239 molecular clone has been subcloned into p239SpSp5′ and p239SpE3′/nef-open, which together yield a full-length provirus upon ligation at a common SphI site (28). Expression constructs for the HIV-1 and SIVmac Gag polyproteins were based on HXBH10-PR− (27), a protease-defective variant of HXBH10, and HXBH10/SIVgag (3), a variant of HXBH10 that contains the gag gene of SIVmac239 in place of HIV-1 gag and pol sequences. HIV-1 sequences were introduced into the CA coding region of the SIVmac239 gag gene by oligonucleotide-directed mutagenesis as described previously (29). Individual codons in the CA coding region of the HIV-1 gag gene were also changed by oligonucleotide-directed mutagenesis. The desired mutations were transferred into p239SpSp5′ and HXBH10/SIVgag on NarI–BspHI fragments (nucleotides 823-2783 of SIVmac239) and into HXBH10 and HXBH10-PR− on BssHII–ApaI fragments (nucleotides 710-2009 of HXBH10). In each case, the presence of the expected sequence was confirmed by DNA sequence analysis.
Viral Protein Analysis.
HeLa cells were grown in DMEM with 10% fetal calf serum (FCS) and transfected with HIV-1 and SIVmac Gag polyprotein expression constructs by a calcium phosphate precipitation technique as described previously (30). The cultures were metabolically labeled with [35S]methionine (50 μCi/ml; 1 Ci = 37 GBq) for 48–60 hr posttransfection. Virions released into the supernatant were pelleted through a 20% sucrose cushion (in PBS) for 90 min at 4°C and 27,000 rpm in a Beckman SW 41 rotor. Pelleted viral particles were lysed in RIPA buffer (140 mM NaCl/8 mM Na2HPO4/2 mM NaH2PO4/1% Nonidet P-40/0.5% sodium deoxycholate/0.05% SDS) and either directly analyzed by SDS/PAGE or by immunoprecipitation with a rabbit anti-human cyclophilin A antiserum prior to SDS/PAGE (3).
Virus Replication Studies.
HIV-1 virus stocks were produced by transient transfection of HeLa cells. At 3 days posttransfection, supernatants were collected and passed through 0.45-μm syringe filters. Virus corresponding to 2 × 104 cpm of reverse transcriptase (RT) activity measured as described (31) was used to infect 5 × 106 CEMx174 cells. To explore the ability of proviral SIVmac constructs to initiate a productive infection, CEMx174 cells were transfected with the DEAE–dextran method using wild-type or mutant p239SpSp5′ ligated to p239SpE3′/nef-open at a common SphI site as described (32). The transfected CEMx174 cultures were maintained in RPMI 1640 medium supplemented with 10% FCS, and RT activity in the culture supernatants was measured at regular intervals. Cell-free supernatant containing 2 × 104 cpm of RT activity was used to infect 5 × 106 fresh CEMx174 cells. The cells were exposed to virus-containing supernatant for 1 hr, and the cultures were then split and maintained in the absence or presence of SDZ NIM 811 at 0.5 μM. Virus replication after infection was monitored by measuring RT activity in the culture supernatants.
RESULTS
Transfer of the Primary CyPA-Binding Site Confers Cyclosporin Sensitivity to SIVmac.
It was shown previously that a SIVmac/HIV-1 gag chimera termed SHS encodes a protein capable of binding CyPA (2). The SHS gag chimera harbors a 88-nt PstI–SpeI fragment from the HIV-1 CA coding region in a SIVmac background (2). An alignment of the sequence encoded by the 88-nt HIV-1 fragment with the corresponding sequence from SIVmac and other primate lentiviruses reveals a highly variable region centered around Pro-90 of HIV-1 CA (2). Single amino acid substitutions in this region showed that Pro-90 is critical for the incorporation of CyPA into HIV-1 virions (2, 18). Because SIVmac has a proline at the equivalent position of CA, we examined whether differences in the region surrounding the proline account for the inability of the SIVmac Gag polyprotein to interact with CyPA. To this end, we generated the S/H90–93 gag gene, which encodes a SIVmac Gag polyprotein that harbors residues just C-terminal to the critical proline from HIV-1 CA (Fig. 1A). Additionally, the coding sequence for an 8-aa segment centered around Pro-90 of HIV-1 CA was transferred to the corresponding position of the SIVmac gag gene, yielding the S/H86–93 gag gene (Fig. 1A).
Figure 1.
(A) Amino acid sequence alignment between the parental and chimeric Gag polyproteins. The loop region that connects α-helices IV and V of HIV-1 CA (22, 23) is shown. Numbering is with respect to the N termini of the SIVmac239 and HIV-1HXB2 CA domains. Conserved positions are indicated by dashes and sequence gaps are indicated by dots. HIV-1 CyPA-binding-site residues Val-86 to Pro-93 (24, 25) as well as CyPA-binding-site residues transferred to the SIVmac Gag polyprotein are boxed. The position of the proline critical for CyPA binding is indicated by an arrow. HIV-1 residues involved in the formation of a type II tight turn (22) are underlined. The alanine in the S/H86–90A chimera that was introduced to fill a gap in the alignment with HIV-1 is in bold. (B) Detailed view of the HIV-1 CyPA-binding loop produced with molscript (33). The hydrogen bond between Ala-92 and Gln-95 is indicated.
To determine the effects of these modifications on the incorporation of CyPA, constructs designed to express the parental and chimeric gag genes were transfected into HeLa cells. Virions released during metabolic labeling with [35S]methionine were partially purified by ultracentrifugation through 20% sucrose, disrupted in RIPA buffer, and aliquots were analyzed directly by SDS/PAGE and in parallel by immunoprecipitation with anti-CyPA serum.
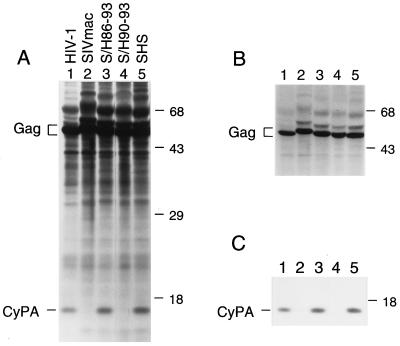
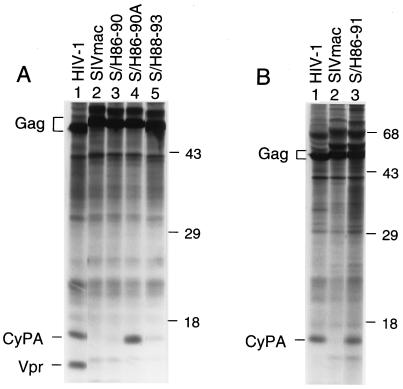
As expected, CyPA was present in particles formed by the HIV-1 and SHS Gag polyproteins but not in particles formed by the authentic Gag polyprotein of SIVmac (Fig. 2). The chimeric S/H90–93 and S/H86–93 gag gene constructs produced similar amounts of viral particles as the parental SIVmac Gag polyprotein expression construct and about twice as much as the HIV-1 Gag polyprotein expression construct (Fig. 2). CyPA was not detected in particles produced by the S/H90–93 gag gene construct (Fig. 2, lane 4). In contrast, particles produced by the S/H86–93 gag gene construct contained CyPA at levels comparable to the level found in HIV-1 virions (Fig. 2, lane 3).
Figure 2.
The transfer of HIV-1 CA residues Val-86 through Pro-93 to the corresponding position of the SIVmac Gag polyprotein induces CyPA incorporation. HeLa cells were transfected with expression constructs for the indicated parental and chimeric Gag polyproteins. [35S]Methionine-labeled viral particles released into the supernatant were pelleted through 20% sucrose and disrupted in RIPA buffer. Aliqouts were either analyzed directly by SDS/PAGE and autoradiography (A) or immunoprecipitated with rabbit anti-CyPA serum prior to SDS/PAGE (C). B shows a shorter autoradiographic exposure of A for the positions occupied by Gag polyproteins. The positions of migration of molecular mass markers (in kilodaltons) are indicated on the right. The positions of Gag polyproteins and of CyPA are indicated on the left.
We next generated full-length SIVmac proviruses harboring the chimeric gag genes and transfected these DNAs into CEMx174 cells, which are highly permissive both for SIVmac and for HIV-1. Although proviral DNA containing the SHS gag gene did not establish a productive infection, significant increases in RT activity were detected after transfection of proviruses that harbored the S/H90–93 and S/H86–93 gag genes (data not shown). Virus was harvested from these cultures as well as from cultures transfected with the parental HIV-1 and SIVmac proviruses, and fresh CEMx174 cells were infected with equivalent amounts of RT activity and kept in the absence or presence of SDZ NIM 811.
As shown in Fig. 3, the parental viruses and the chimeras grew with comparable kinetics, although wild-type SIVmac and HIV-1 reached somewhat higher peak levels of RT activity. As previously reported (3), HIV-1 replicates with significantly delayed kinetics in the presence of 0.5 μM SDZ NIM 811, whereas wild-type SIVmac is resistant to this compound (Fig. 3 A and B). The S/H90–93 chimera, which did not interact with CyPA, was unaffected by SDZ NIM 811 (Fig. 3C). In contrast, the S/H86–93 chimera, which efficiently incorporated CyPA into virions, was as sensitive to SDZ NIM 811 as HIV-1 (Fig. 3D). Thus, the transfer of HIV-1 CA residues 86–93 was sufficient to create a functional CyPA incorporation motif in the CA domain of SIVmac CA and to confer a cyclosporin-sensitive phenotype.
Figure 3.
Transfer of the primary CyPA-binding site to SIVmac confers cyclosporin sensitivity. Viral replication kinetics were compared in CEMx174 cells infected with the parental viruses HIV-1HXB2 (A) and SIVmac239 (B), or the SIVmac-based chimeras S/H90–93 (C) and S/H86–93 (D). All infections were done for 1 hr using CEMx174 cell-derived virus stocks normalized to 2 × 104 cpm RT activity. Cultures were then split and maintained in the absence of drug (○) or in the presence of 0.5 μM SDZ NIM 811 (•). Viral replication was monitored daily by measuring particle-associated RT activity in the culture supernatants.
Scanning Mutagenesis of the HIV-1 CyPA-Binding Site.
The experiments described above provide in vivo evidence that determinants for CyPA binding are located between residues 86 and 93 of HIV-1 CA. To examine the relative importance of single residues within this region for CyPA incorporation and HIV-1 replication, HIV-1 CA codons 86–93 were mutated individually. Ala-88 and Ala-92 were changed to glycine, and all other residues were replaced by alanine. The mutations were introduced into a proviral expression construct for the HIV-1 Gag polyprotein Pr55gag, and viral particles were produced by transfection into HeLa cells. As judged from the amount of pelletable Pr55gag in culture supernatants, the A88G mutation reduced particle yield by about 2- to 3-fold; the other mutants produced particles with an efficiency close to that of the parental construct. Particle-associated CyPA levels were only moderately affected by the V86A, H87A, I91A, A92G, and P93A mutations (Fig. 4A). However, the A88G, G89A, and P90A mutations reduced the amount of particle-associated CyPA more than 10-fold (Fig. 4A).
Figure 4.
Effects of single amino acid substitutions in the CyPA-binding site on CyPA incorporation and HIV-1 replication. (A) Comparison of proteins associated with viral particles formed by wild-type (lane 10) and mutant (lanes 1–8) HIV-1 Gag polyproteins or by the Gag polyprotein of SIVmac (lane 9). The mutations introduced into the HIV-1 CA domain are indicated above lanes 1–8. Gag polyprotein expression constructs were transfected into HeLa cells, and [35S]methionine-labeled viral particles were pelleted through sucrose cushions and analyzed directly by SDS/PAGE. (B) Comparison of the replication kinetics of wild-type (WT) HIV-1 and of the indicated HIV-1 CA mutants. CEMx174 cells were infected with equivalent amounts of HeLa cell-derived wild-type or mutant virus, and virus replication was monitored by measuring RT activity in the culture supernatants.
To examine the importance of the mutated residues for virus replication, HIV-1 proviruses that differ from the parental proviral clone only by the presence of point mutations in the CyPA-binding site were constructed. Virus stocks normalized for particle-associated RT activity were generated by transfection of proviral DNAs into HeLa cells and used to infect CEMx174 cells. Mutants A88G, G89A, and P90A, which exhibited marked defects in CyPA incorporation, replicated poorly in CEMx174 cells (Fig. 4B Upper), whereas the other five mutants replicated essentially with wild-type kinetics (Fig. 4B Lower). Thus, the effects of the single amino acid substitutions on CyPA incorporation correlated closely with their effects on HIV-1 replication.
N-Terminal Portions of the Primary CyPA-Binding Site Can Confer Cyclosporin Dependence.
To further characterize the relationship between determinants required for CyPA incorporation and those involved in the anti-viral effect of CyPA-binding compounds, we transferred various portions of the HIV-1 CyPA-binding site to the corresponding position in SIVmac. The S/H88–93 gag gene was constructed to determine the contribution of CyPA-binding-site residues proximal to the critical Ala-88. The CyPA-binding-site residues encoded by the S/H88–93 gag gene include the three critical interface residues Ala-88, Gly-89, and Pro-90 as well as interface residues distal to Pro-90 (Fig. 1A). Normalized for Gag, particles produced upon expression of the S/H88–93 gag gene in HeLa cells contained about 10-fold less CyPA than HIV-1 virions or chimeric particles produced by the S/H86–93 gag gene construct (Fig. 5A). A full-length SIVmac provirus harboring the S/H88–93 gag gene was able to initiate a productive infection after transfection into CEMx174 cells, and passage in the presence or absence of SDZ NIM 811 showed that the S/H88–93 chimera is unaffected by this compound (Fig. 6A).
Figure 5.
CyPA incorporation into SIVmac particles that harbor different portions of the HIV-1 CyPA-binding site. HeLa cells transfected with Gag polyprotein expression constructs were metabolically labeled with [35S]methionine, and the protein content of sucrose-purified viral particles was compared by SDS/PAGE. The parental and chimeric gag genes expressed are indicated above each lane. A and B show the results of separate experiments.
Figure 6.
The presence of a functional CyPA incorporation site in SIVmac can confer cyclosporin dependence. CEMx174 cells were infected for 1 hr with SIVmac-based chimeras S/H88–93 (A), S/H86–90 (B), S/H86–90A (C), or S/H86–91 (D) using CEMx174 cell-derived virus stocks normalized to 2 × 104 cpm RT activity. Viral replication kinetics in the absence of drug (○) or in the presence of 0.5 μM SDZ NIM 811 (•) were then monitored by measuring RT activity in the culture supernatants. In case of the S/H86–90A chimera (C), virus replication in the presence of 0.5 μM CsH (▴) was also examined.
To determine whether CyPA-binding-site residues distal to the critical Pro-90 contribute to the cyclosporin-sensitive phenotype, we inserted HIV-1 CA residues 86–90 between residues 84 and 89 of the SIVmac CA domain as illustrated in Fig. 1A. An expression construct for the resulting S/H86–90 gag gene efficiently produced viral particles in HeLa cells; however, the chimeric particles did not contain CyPA at detectable levels (Fig. 5A, lane 3). A full-length SIVmac provirus containing the S/H86–90 gag gene initiated a productive infection after transfection into CEMx174 cells. Passage after normalization for RT activity showed that the chimera replicates with delayed kinetics (Fig. 6B) relative to authentic SIVmac (Fig. 3B). The S/H86–90 chimera replicated with similar efficiency in the presence or absence of 0.5 μM SDZ NIM 811 (Fig. 6B), consistent with its inability to incorporate CyPA.
The failure of the S/H86–90 chimera to incorporate CyPA was unexpected, because this construct harbored all of the HIV-1 CyPA-binding-site residues identified as critical by scanning mutagenesis. Therefore, we considered the possibility that the length of the CyPA-binding loop is critical for the interaction between CA and CyPA. An optimal alignment of HIV-1 and SIVmac CA sequences requires the insertion of two gaps into the SIVmac sequence at the position corresponding to the CyPA-binding loop; one of these gaps remains in an alignment of HIV-1 and the S/H86–90 chimera (Fig. 1A). To fill this gap, we inserted an alanine into the S/H86–90 CA domain as illustrated in Fig. 1A. Remarkably, this modification led to the incorporation of CyPA at levels that were at least as high as those found in HIV-1 virions (Fig. 5A, lane 4).
Surprisingly, transfection of a full-length SIVmac provirus containing the S/H86–90A gag gene into CEMx174 cells resulted in virus replication only when the transfected cells were kept in the presence of SDZ NIM 811 (data not shown). Cell-free supernatant from the latter culture was incubated with fresh CEMx174 cells after normalization for RT activity, and the cells were then split and maintained in the absence of drug or in the presence of 0.5 μM SDZ NIM 811 or CsH. In the presence of SDZ NIM 811, the chimera exhibited only moderately delayed replication kinetics (Fig. 6C) compared with those of wild-type SIVmac (Fig. 3B). In contrast, virus replication in the parallel culture, which was kept in the absence of drug, was markedly delayed (Fig. 6C). Unlike SDZ NIM 811, the CsA analogue CsH, which has an extremely low affinity for CyPA (6), did not accelerate virus replication (Fig. 6C). Evidently, the S/H86–90A chimera was dependent on the presence of the cyclophilin-binding compound SDZ NIM 811 for efficient virus replication.
The cyclosporin-sensitive S/H86–93 chimera and the cyclosporin-dependent S/H86–90A chimera both harbor the primary HIV-1 CyPA-binding site up to Pro-90 (Fig. 1A). However, these constructs differ in a region distal to Pro-90 that forms a type II tight turn in the three-dimensional structure of HIV-1 CA (Fig. 1B) (22). Although the S/H86–93 chimera is identical to HIV-1 throughout this region, only two of the four residues involved in the formation of the type II tight turn are present in the S/H86–90A chimera (Fig. 1). To further investigate whether the type II turn plays a role in the effect of SDZ NIM 811, we generated a chimera that harbors all HIV-1 CyPA-binding-site residues except for those involved in the formation of the type II turn. To this end, HIV-1 CA residues 86–91 were transferred to the corresponding position of SIVmac. In an effort to preserve the length of the CyPA-binding loop, the HIV-1 sequence was inserted between residues 84 and 89 of the SIVmac CA domain as illustrated in Fig. 1A.
As expected, particles formed by an expression construct for the S/H86–91 gag gene efficiently incorporated CyPA (Fig. 5B, lane 3). CEMx174 cells transfected with a full-length SIVmac provirus harboring the S/H86–91 gag gene showed evidence for virus replication when kept in the presence of SDZ NIM 811. Passage after normalization for RT activity revealed that, as in the case of the S/H86–90A chimera, the ability of the S/H86–91 chimera to replicate efficiently was dependent on the presence of SDZ NIM 811 (Fig. 6D). We conclude that the presence or absence of two residues that participate in the formation of a type II tight turn just distal to the CyPA-binding site determined whether virus replication was inhibited or enhanced by SDZ NIM 811.
DISCUSSION
The results reported here are consistent with the recently solved crystal structures of CyPA bound to the N-terminal domain of HIV-1 CA or to a 25-mer HIV-1 CA fragment, which show that CA residues 86–93 bind to CyPA (24, 25). These residues form part of an exposed loop that connects helices IV and V of HIV-1 CA (22, 23). Our results imply that residues 86–93 of HIV-1 CA control not only CyPA binding but also the effect of CyPA on virus replication.
It has been shown previously that HIV-1 CA residues Gly-89 and Pro-90 are crucial for CyPA binding and HIV-1 replication (2, 18). Our scanning mutagenesis indicates that Ala-88 is equally important for the incorporation of CyPA into HIV-1 virions, whereas individual residues proximal to Ala-88 or distal to Pro-90 contribute only moderately. The effects of single amino acid substitutions on CyPA incorporation correlated closely with their effects on HIV-1 replication, because Ala-88, Gly-89, and Pro-90 were the only residues in the primary CyPA-binding site whose substitution significantly reduced virus replication. Interestingly, in the CA–CyPA cocrystal, Ala-88, Gly-89, and Pro-90 are the CA interface residues that are buried in the CyPA active-site groove (24).
Our results indicate that the two residues just N-terminal to the Ala-Gly-Pro motif, although not critical at the individual level, together contribute significantly to the interaction with CyPA and to the cyclosporin-sensitive phenotype. Additionally, the length of the loop between helices IV and V appears to be important for CyPA incorporation. An optimal alignment between the CA domains of HIV-1 and SIVmac requires the introduction of two gaps into the SIVmac sequence within the region that corresponds to the primary CyPA-binding site of HIV-1. We find that HIV-1 CyPA-binding-site residues distal to the critical Pro-90 need not be transferred to SIVmac to achieve efficient incorporation of CyPA, provided that no gaps remain in the alignment with HIV-1. One of these gaps could be filled by an unrelated alanine residue that is unlikely to contribute to the sequence-specific binding of CyPA.
In particular, contact residues that participate in the formation of a type II tight turn within the HIV-1 CyPA-binding loop were not required to transfer the ability to incorporate CyPA. However, the presence or absence of these residues determined whether SDZ NIM 811 inhibited or enhanced the replication of SIVmac mutants capable of incorporating CyPA. Both effects of SDZ NIM 811 were dependent on the presence of a functional CyPA-binding site in the CA domain of SIVmac, indicating that the drug acted through CyPA. Our results suggest that the type II tight turn formed by HIV-1 CA residues 92–95 (22), although dispensable for CyPA incorporation, is crucial for the effect of CyPA on virus replication.
The phenotype of the cyclosporin-dependent SIVmac mutants is reminiscent of that of previously described HIV-1 variants that were obtained by serial passage in the presence of SDZ NIM 811 (20). Depending on the cell line in which they were propagated, these HIV-1 variants exhibited a cyclosporin-resistant or even a cyclosporin-dependent phenotype (20, 21). Interestingly, the cyclosporin-resistant/dependent phenotype was conferred by either of two single amino acid substitutions in CA (20, 21), which are expected to destabilize the type II tight turn in the CyPA-binding loop (22). The substitutions did not affect the ability of the variants to interact with CyPA but apparently eliminated the requirement for the interaction (21).
On the basis of the packing of CA and CyPA in the cocrystal, a model was recently proposed to explain the enhancement of HIV-1 virion infectivity by CyPA (24, 26). In this model, virion-associated CyPA would interfere with CA–CA interactions that involve the CyPA-binding loop, in particular, the type II tight turn just C-terminal to the primary CyPA-binding site. CyPA would thereby destabilize the virion core and facilitate uncoating, which is consistent with the reported role of CyPA early in the viral life cycle (18). Evidently, the cyclosporin-sensitive as well as the cyclosporin-dependent phenotypes of SIVmac mutants described in the present report could also be explained by this model. Because SIVmac is not adapted to the presence of a CyPA-binding site, CA–CA interactions in the cyclosporin-sensitive S/H86–90A and S/H86–91 chimeras may have been too weak to tolerate interference by CyPA. That the interaction with CyPA, rather than the transfer of HIV-1 CyPA-binding-site residues per se, interfered with the function of SIVmac CA is strongly suggested by the observation that the mutants could be rescued by SDZ NIM 811, but not by CsH, a CsA analogue with negligible affinity for CyPA (6). A role of the type II tight turn in the CyPA-binding loop in core stability may explain our observation that the effect of SDZ NIM 811 was reversed when this region was included in the sequence transferred to SIVmac.
Although previous studies have identified CA as a functional target of CyPA, the relationship between determinants within CA that mediate the interaction with CyPA and those involved in cyclosporin sensitivity remained poorly defined. Our study establishes that the transfer of the primary CyPA-binding site to SIVmac is sufficient to confer an HIV-1-like sensitivity to a CyPA-binding compound. The results strongly suggest that this element not only provides a docking site for CyPA but also specifies the requirement for the interaction with CyPA for efficient virus replication.
Acknowledgments
We thank Christopher Walsh for providing antiserum against CyPA, Sandoz Pharma AG for providing SDZ NIM 811 and CsH, Christopher Hill for providing the coordinates for the CA–CyPA cocrystal, and Michael Farzan for help with molscript. This work was supported by National Institutes of Health Grants AI29873, AI28691 (Center for AIDS Research), and CA06516 (Cancer Center) and by a gift from the G. Harold and Leila Y. Mathers Charitable Foundation.
ABBREVIATIONS
- CyPA
cyclophilin A
- CsA
cyclosporin A
- SIVmac
simian immunodeficiency virus from Macaca mulatta
- CA
capsid
- Pr55gag
HIV-1 Gag polyprotein
- RT
reverse transcriptase
References
- 1.Luban J, Bossolt K L, Franke E K, Kalpana G V, Goff S P. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 2.Franke E K, Yuan H E H, Luban J. Nature (London) 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 3.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C, Sodroski J, Göttlinger H G. Nature (London) 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 4.Ott D E, Coren L V, Johnson D G, Sowder R C, Arthur L O, Henderson L E. AIDS Res Hum Retroviruses. 1995;11:1003–1006. doi: 10.1089/aid.1995.11.1003. [DOI] [PubMed] [Google Scholar]
- 5.Dorfman T, Göttlinger H G. J Virol. 1996;70:5751–5757. doi: 10.1128/jvi.70.9.5751-5757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handschumacher R, Harding M, Rice J, Drugge R. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 7.Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid F X. Nature (London) 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N, Hayano T, Suzuki M. Nature (London) 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 9.Walsh C T, Zydowsky L D, McKeon F D. J Biol Chem. 1992;267:13115–13118. [PubMed] [Google Scholar]
- 10.Schreiber S L. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 11.Kallen J, Spitzfaden C, Zurini M G M, Wider G, Widmer H, Wüthrich K, Walkinshaw M D. Nature (London) 1991;353:276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- 12.Montague J W, Hughes F M, Cidlowski J A. J Biol Chem. 1997;272:6677–6684. doi: 10.1074/jbc.272.10.6677. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, Schreiber S L. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 14.Sigal N H, Dumont F, Durette P, Siekierka J, Peterson L, Rich D H, Dunlap B E, Staruch M J, Melino M R, Koprak S L, Williams D, Witzel B, Pisano J M. J Exp Med. 1991;173:619–628. doi: 10.1084/jem.173.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenwirth B, Billich A, Datema R, Donatsch P, Hammerschmid F, Harrison R, Hiestand P, Jaksche H, Mayer P, Peichl P, Quesniaux V, Schatz F, Schuurman H-J, Traber R, Wenger R, Wolff B, Zenke G, Zurini M. Antimicrob Agents Chemother. 1994;38:1763–1772. doi: 10.1128/aac.38.8.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartz S R, Hohenwalter E, Hu M-K, Rich D H, Malkovsky M. Proc Natl Acad Sci USA. 1995;92:5381–5385. doi: 10.1073/pnas.92.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franke E K, Luban J. Virology. 1996;222:279–282. doi: 10.1006/viro.1996.0421. [DOI] [PubMed] [Google Scholar]
- 18.Braaten D, Franke E K, Luban J. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinkasserer A, Harrison R, Billich A, Hammerschmid F, Werner G, Wolff B, Peichl P, Palfi G, Schnitzel W, Mlynar E, Rosenwirth B. J Virol. 1995;69:814–824. doi: 10.1128/jvi.69.2.814-824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aberham C, Weber S, Phares W. J Virol. 1996;70:3536–3544. doi: 10.1128/jvi.70.6.3536-3544.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braaten D, Aberham C, Franke E K, Yin L, Phares W, Luban J. J Virol. 1996;70:5170–5176. doi: 10.1128/jvi.70.8.5170-5176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gitti R K, Lee B M, Walker J, Summers M F, Yoo S, Sundquist W I. Science. 1996;273:231–235. doi: 10.1126/science.273.5272.231. [DOI] [PubMed] [Google Scholar]
- 23.Momany C, Kovari L C, Prongay A J, Keller W, Gitti R K, Lee B M, Gorbalenya A E, Tong L, McClure J, Ehrlich L S, Summers M F, Carter C, Rossmann M G. Nat Struct Biol. 1996;3:763–770. doi: 10.1038/nsb0996-763. [DOI] [PubMed] [Google Scholar]
- 24.Gamble T R, Vajdos F F, Yoo S, Worthylake D K, Houseweart M, Sundquist W I, Hill C P. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Chen Y, Schutkowski M, Fischer G, Ke H. Structure. 1997;5:139–146. doi: 10.1016/s0969-2126(97)00172-x. [DOI] [PubMed] [Google Scholar]
- 26.Luban J. Cell. 1996;87:1157–1159. doi: 10.1016/s0092-8674(00)81811-5. [DOI] [PubMed] [Google Scholar]
- 27.Göttlinger H G, Dorfman T, Cohen E A, Haseltine W A. Proc Natl Acad Sci USA. 1993;90:7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 29.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 30.Cullen B R. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 31.Dayton A I, Sodroski J S, Rosen C A, Goh W C, Haseltine W A. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. J Acquired Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 33.Kraulis P J. J Appl Cryst. 1991;24:946–950. [Google Scholar]