Abstract
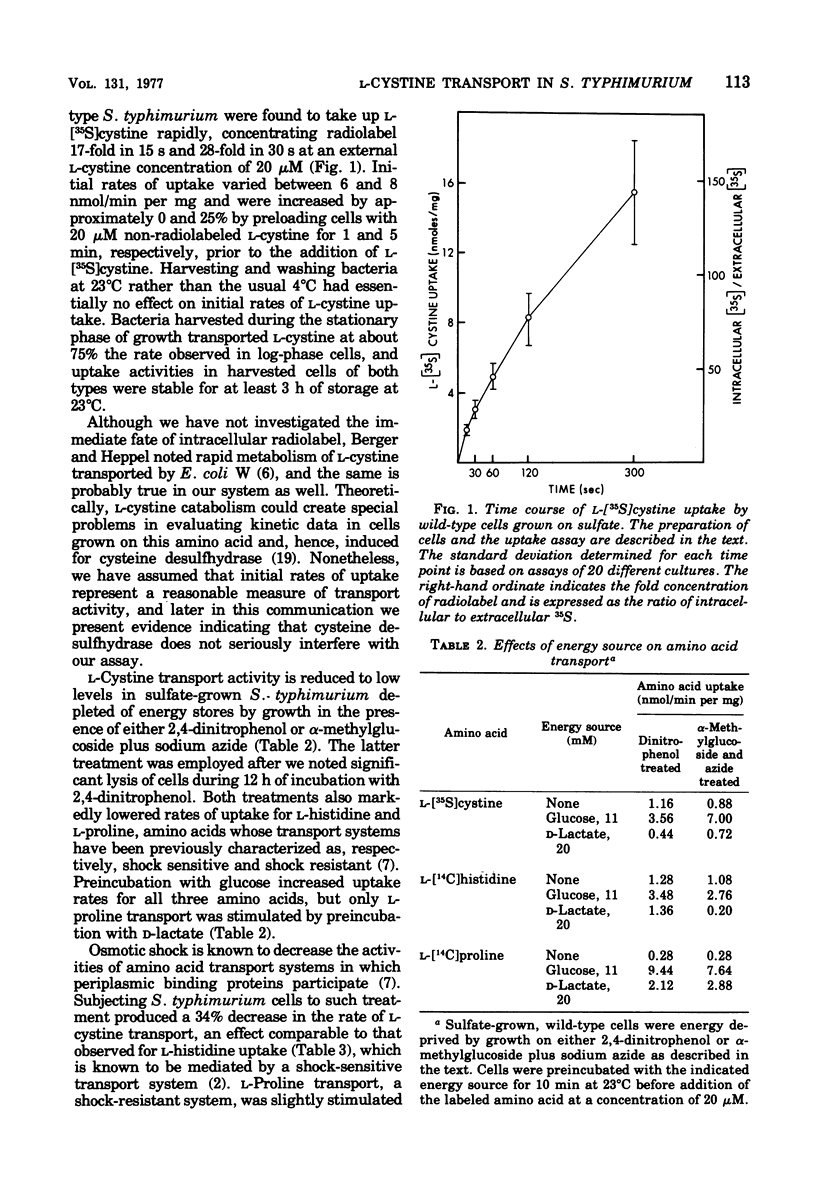
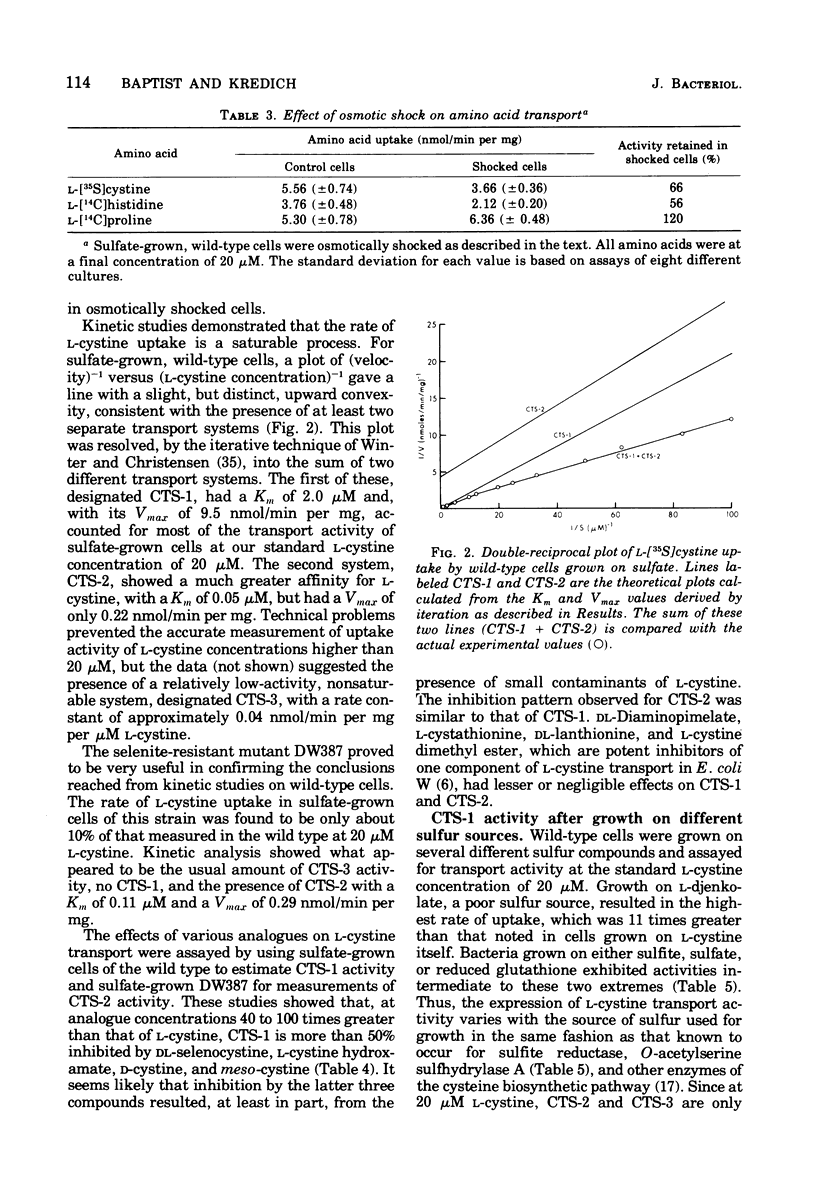
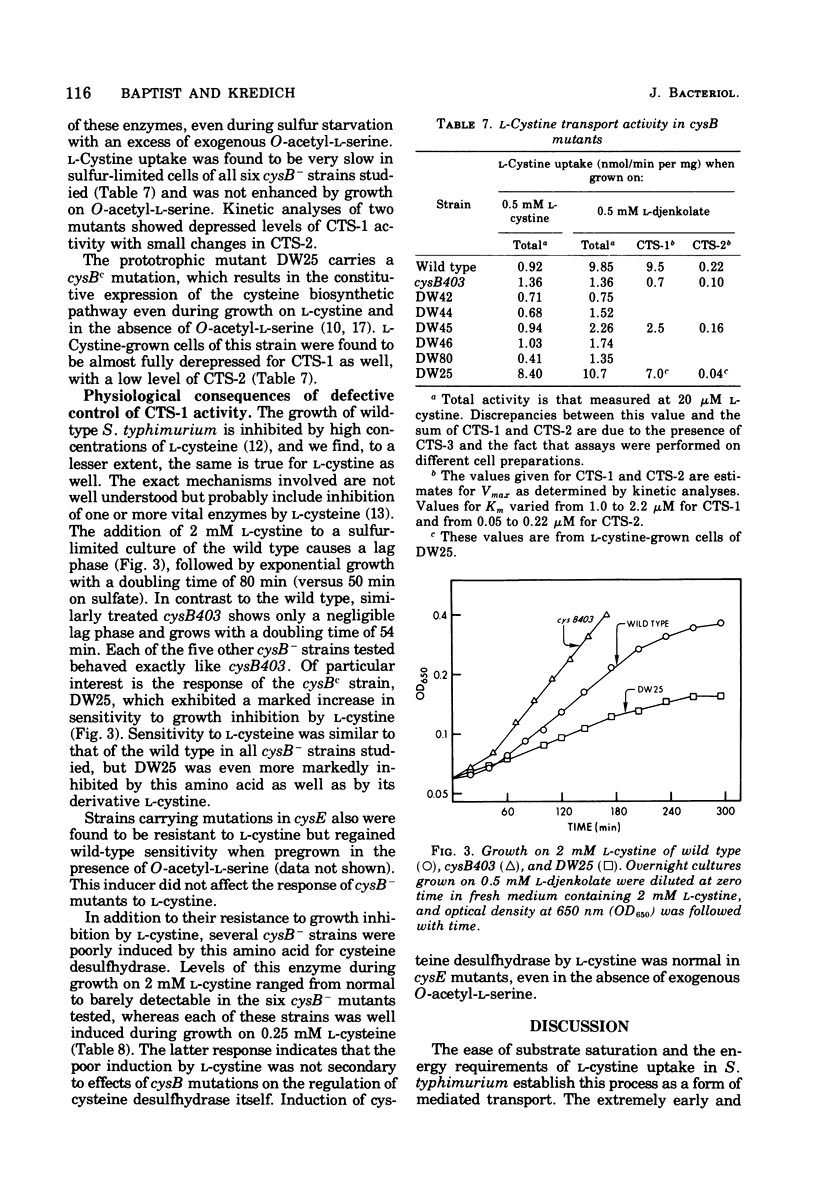
A kinetic analysis of L-cystine uptake in wild-type Salmonella typhimurium indicates the presence of at least two, and possibly three, separate transport systems. CTS-1 accounts for the majority of uptake at 20 muM L-cystine, with a Vmax of 9.5 nmol/min per mg and a Km of 2.0 muM; CTS-2 is a low-capacity, higher-affinity system with a Vmax of 0.22 nmol/min per mg and a Km of 0.05 muM; a third, nonsaturable process has been designated CTS-3. We find that wild-type CTS-1 levels are at least 11 times higher in sulfur-limited cells than in L-cystine-grown cells. Pleiotropic cysteine auxotrophs of the types cysE (lacking serine transacetylase) and cysB- (lacking a regulatory element of positive control) have very low levels of CTS-1 even when grown under conditions of sulfur limitation, which response is analogous to that previously observed for cysteine biosynthetic enzymes (N . M. Kredich, J. Biol. Chem. 246:3474-3484, 1971). CTS-1 is induced in cysE mutants by growth in the presence of O-acetyl-L-serine (the product of serine transacetylase), again paralleling the behavior of the cysteine biosynthetic pathway. Strain DW25, a prototrophic cysBc mutant, which is constitutive for cysteine biosynthesis, is also derepressed for CTS-1 when grown on L-cystine. Since CTS-1 is regulated by sulfur limitation, O-acetyl-L-serine, and the cysB gene product, the same three conditions controlling cysteine biosynthesis, we propose that this transport system is a part of the cysteine regulon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNSTEIN H. R. Synthesis of alpha-methyl- and beta-methyl-DL-cystine. Biochem J. 1958 Feb;68(2):333–338. doi: 10.1042/bj0680333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrell J. W., Kaufman E. E., Lipsett M. N. The biosynthesis of 4-thiouridylate. Separation and purification of two enzymes in the transfer ribonucleic acid-sulfurtransferase system. J Biol Chem. 1971 Jan 25;246(2):294–301. [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. J., Quay S. C., Oxender D. L. Mapping of two loci affecting the regulation of branched-chain amino acid transport in Escherichia coli K-12. J Bacteriol. 1976 Apr;126(1):80–90. doi: 10.1128/jb.126.1.80-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem. 1972 Dec 10;247(23):7684–7694. [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge P. R., Ayling P. D. The regulation of glutamine transport and glutamine synthetase in Salmonella typhimurium. J Gen Microbiol. 1976 Aug;96(2):324–334. doi: 10.1099/00221287-95-2-324. [DOI] [PubMed] [Google Scholar]
- Cheney R. W., Jr, Kredich N. M. Fine-structure genetic map of the cysB locus in Salmonella typhimurium. J Bacteriol. 1975 Dec;124(3):1273–1281. doi: 10.1128/jb.124.3.1273-1281.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. M., Wallenstein A., Monty K. J. Regulatory features of the cysteine desulfhydrase of Salmonella typhimurium. Biochim Biophys Acta. 1973 Jun 20;313(1):156–162. doi: 10.1016/0304-4165(73)90196-7. [DOI] [PubMed] [Google Scholar]
- Datta P. Regulation of homoserine biosynthesis by L-cysteine, a terminal metabolite of a linked pathway. Proc Natl Acad Sci U S A. 1967 Aug;58(2):635–641. doi: 10.1073/pnas.58.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S. Genetics of amino acid transport in bacteria. Annu Rev Genet. 1974;8:103–133. doi: 10.1146/annurev.ge.08.120174.000535. [DOI] [PubMed] [Google Scholar]
- Hulanicka M. D., Kredich N. M., Treiman D. M. The structural gene for O-acetylserine sulfhydrylase A in Salmonella typhimurium. Identity with the trzA locus. J Biol Chem. 1974 Feb 10;249(3):867–872. [PubMed] [Google Scholar]
- Kadner R. J. Regulation of methionine transport activity in Escherichia coli. J Bacteriol. 1975 Apr;122(1):110–119. doi: 10.1128/jb.122.1.110-119.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Foote L. J., Hulanicka M. D. Studies on the mechanism of inhibition of Salmonella typhimurium by 1,2,4-triazole. J Biol Chem. 1975 Sep 25;250(18):7324–7331. [PubMed] [Google Scholar]
- Kredich N. M., Foote L. J., Keenan B. S. The stoichiometry and kinetics of the inducible cysteine desulfhydrase from Salmonella typhimurium. J Biol Chem. 1973 Sep 10;248(17):6187–6196. [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Purdy D. R., Koch A. L. Energy cost of galactoside transport to Escherichia coli. J Bacteriol. 1976 Sep;127(3):1188–1196. doi: 10.1128/jb.127.3.1188-1196.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Kline E. L., Oxender D. L. Role of leucyl-tRNA synthetase in regulation of branched-chain amino-acid transport. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3921–3924. doi: 10.1073/pnas.72.10.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L. Regulation of branched-chain amino acid transport in Escherichia coli. J Bacteriol. 1976 Sep;127(3):1225–1238. doi: 10.1128/jb.127.3.1225-1238.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L., Tsuyumu S., Umbarger H. E. Separate regulation of transport and biosynthesis of leucine, isoleucine, and valine in bacteria. J Bacteriol. 1975 Jun;122(3):994–1000. doi: 10.1128/jb.122.3.994-1000.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Tully M., Yudkin M. D. The nature of the product of the cys B gene of Escherichia coli. Mol Gen Genet. 1975;136(2):181–183. doi: 10.1007/BF00272038. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Willis R. C., Iwata K. K., Furlong C. E. Regulation of Glutamine Transport in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1032–1037. doi: 10.1128/jb.122.3.1032-1037.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- Winter C. G., Christensen H. N. Contrasts in neutral amino acid transport by rabbit erythrocytes and reticulocytes. J Biol Chem. 1965 Sep;240(9):3594–3600. [PubMed] [Google Scholar]



