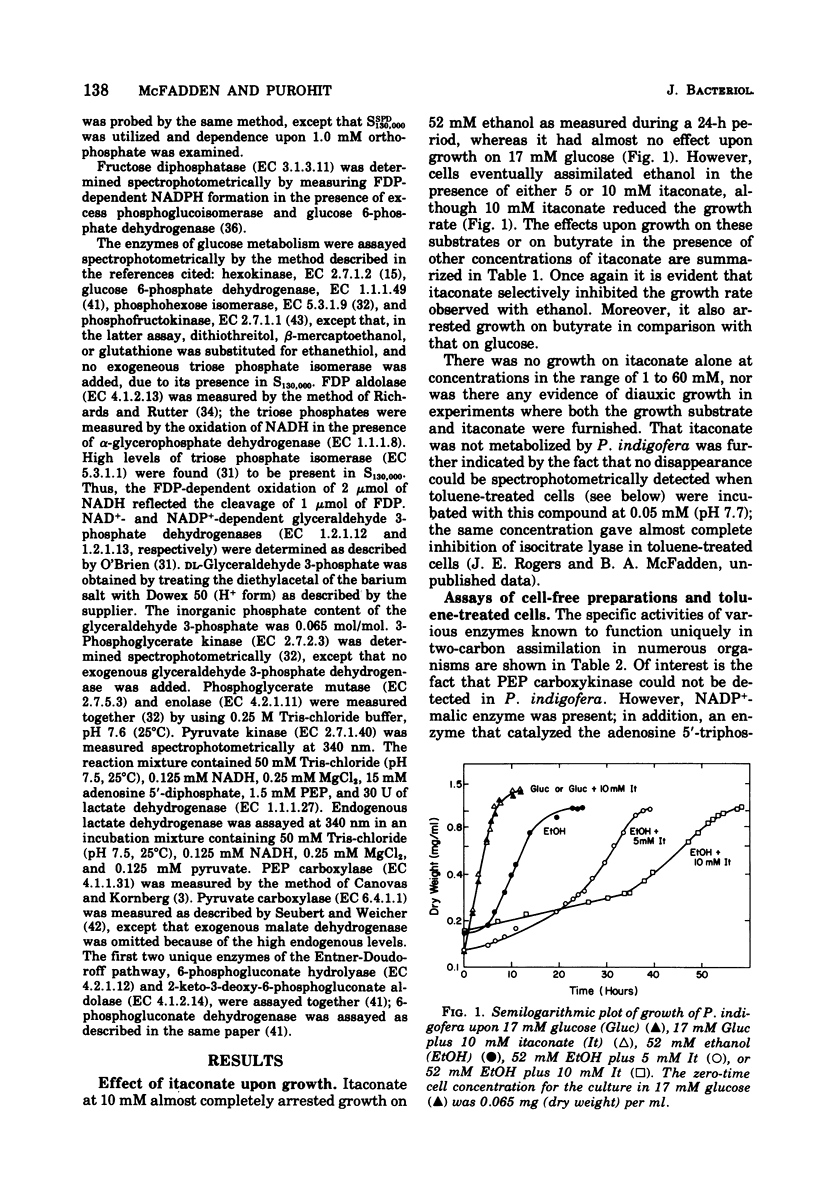
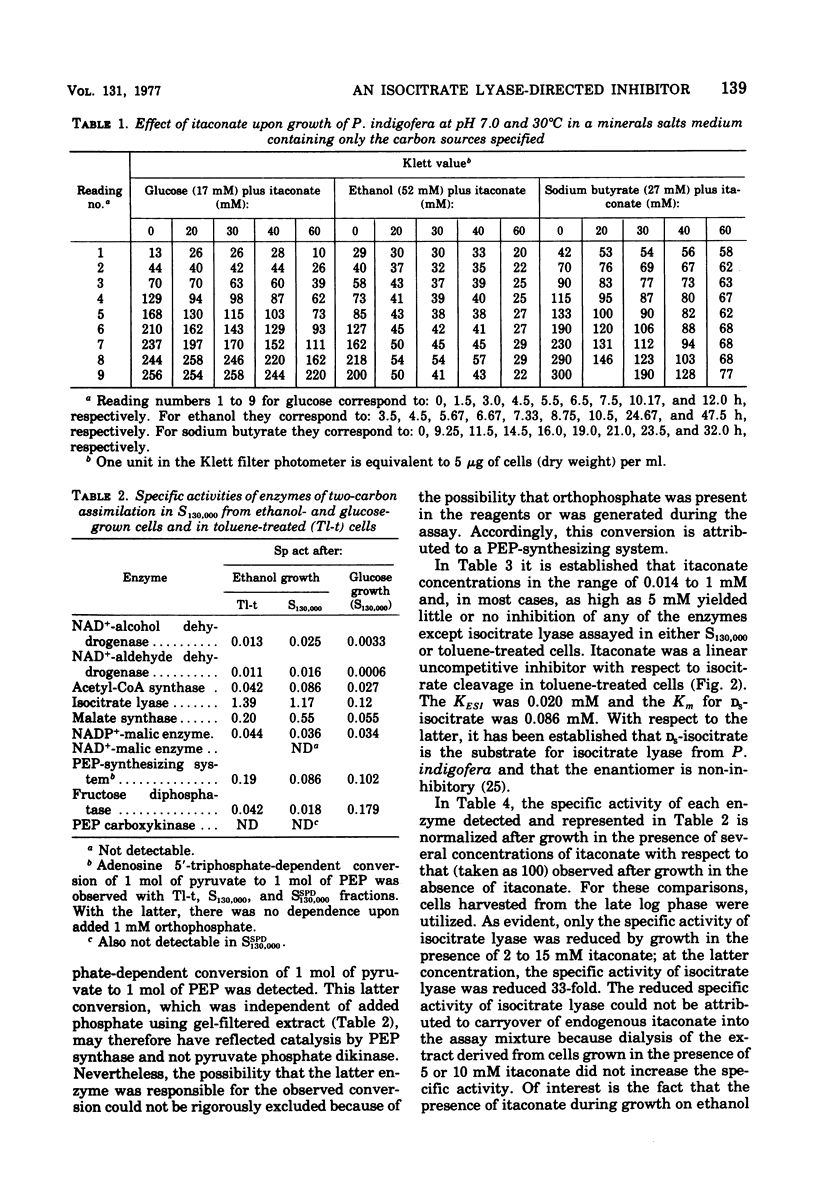
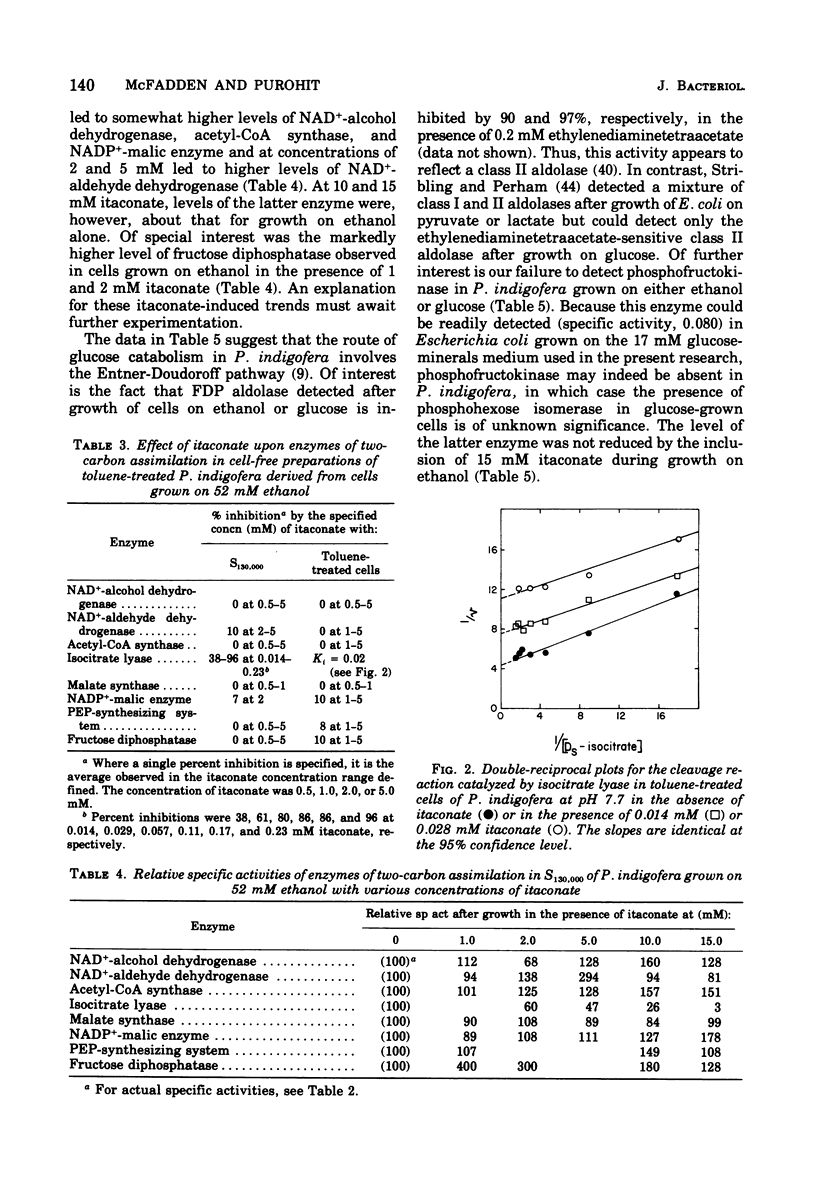
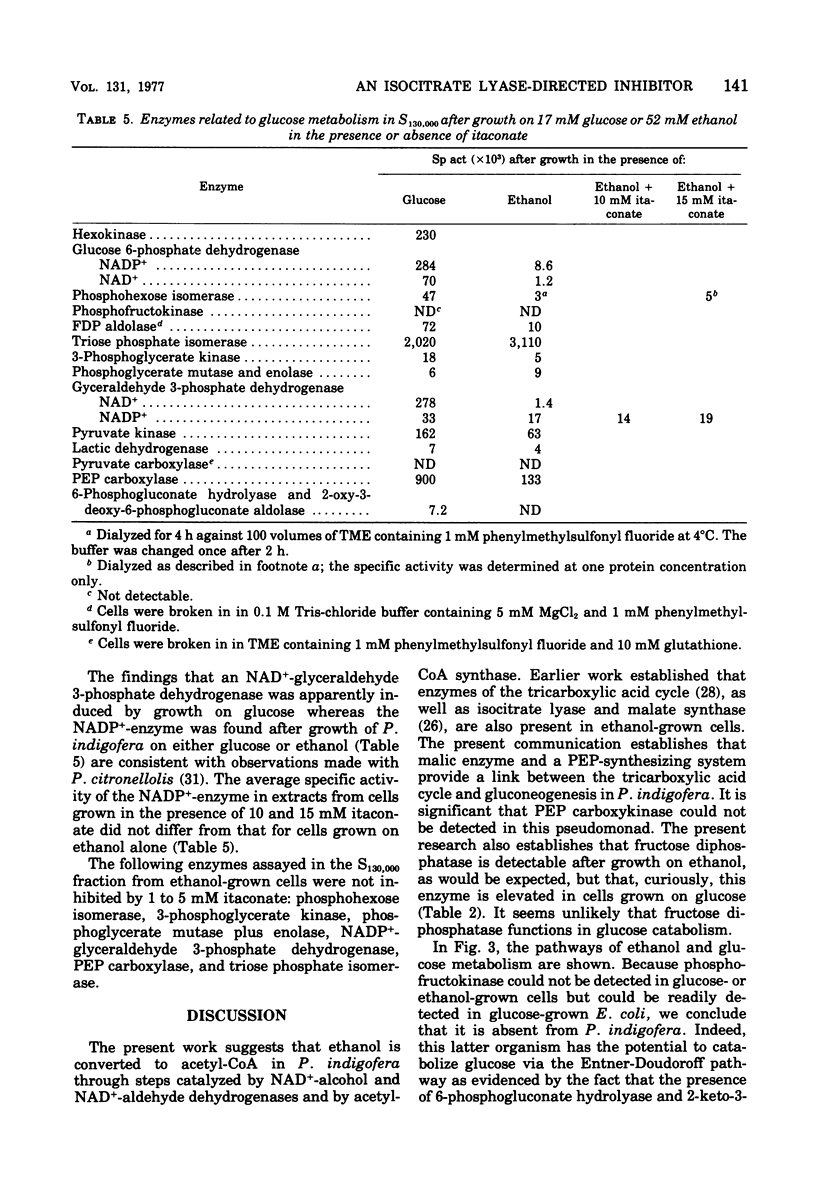
Abstract
Enzymes catalyzing steps from ethanol to acetyl-coenzyme A, from malate to pyruvate, and from pyruvate to glucose 6-phosphate were identified in ethanol-grown Pseudomonas indigofera. Enzymes catalyzing the catabolism of glucose to pyruvate via the Entner-Doudoroff pathway were identified in glucose-grown cells. Phosphofructokinase could not be detected in Pseudomonas indigofera. Itaconate, a potent inhibitor of isocitrate lyase, abolished growth of P. indigofera on ethanol at concentrations that had little effect upon growth on glucose. The date obtained through enzyme analyses and studies of itaconate inhibition with both extracts and toluene-treated cells suggest that itaconate selectively inhibits and reduces the specific activity of isocitrate lyase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- CANOVAS J. L., KORNBERG H. L. FINE CONTROL OF PHOSPHOPYRUVATE CARBOXYLASE ACTIVITY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Jan;96:169–172. doi: 10.1016/0005-2787(65)90624-6. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Colonna W. J., McFadden B. A. Isocitrate lyase from parasitic and free-living nematodes. Arch Biochem Biophys. 1975 Oct;170(2):608–619. doi: 10.1016/0003-9861(75)90156-3. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. Net formation of phosphoenolpyruvate from pyruvate by Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):618–620. doi: 10.1016/0304-4165(65)90374-0. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The utilization of itaconate by Pseudomonas sp. Biochem J. 1964 Apr;91(1):82–91. doi: 10.1042/bj0910082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., FOSTER S. M. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956 Nov;22(2):253–265. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- HOWES W. V., McFADDEN B. A. Isocitrate lyase and malate synthase in Pseudomonas indigofera. I. Suppression and stimulation during growth. J Bacteriol. 1962 Dec;84:1216–1221. doi: 10.1128/jb.84.6.1216-1221.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWES W. V., McFADDEN B. A. Isocitrate lyase and malate synthase in Pseudomonas indigofera. II. Enzyme changes during the phase of adjustment and the early exponential phase. J Bacteriol. 1962 Dec;84:1222–1227. doi: 10.1128/jb.84.6.1222-1227.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. An assat for PEP carboxykinase in crude tissue extracts. Anal Biochem. 1973 Mar;52(1):280–285. doi: 10.1016/0003-2697(73)90350-3. [DOI] [PubMed] [Google Scholar]
- Hsu R. Y., Lardy H. A. Pigeon liver malic enzyme. II. Isolation, crystallization, and some properties. J Biol Chem. 1967 Feb 10;242(3):520–526. [PubMed] [Google Scholar]
- JONES M. E., BLACK S., FLYNN R. M., LIPMANN F. Acetyl coenzyme a synthesis through pyrophosphoryl split of adenosine triphosphate. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):141–149. doi: 10.1016/0006-3002(53)90133-4. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., ELSDEN S. R. The metabolism of 2-carbon compounds by microorganisms. Adv Enzymol Relat Subj Biochem. 1961;23:401–470. doi: 10.1002/9780470122686.ch8. [DOI] [PubMed] [Google Scholar]
- Körting W., Fairbairn D. Anaerobic energy metabolism in Moniliformis dubius (Acanthocephala). J Parasitol. 1972 Feb;58(1):45–50. [PubMed] [Google Scholar]
- Körting W., Fairbairn D. Changes in beta-oxidation and related enzymes during the life cycle of Strongyloides ratti (Nematoda). J Parasitol. 1971 Dec;57(6):1153–1158. [PubMed] [Google Scholar]
- MCFADDEN B. A., HOWES W. V. Pseudomonas indigofera. J Bacteriol. 1961 Jun;81:858–862. doi: 10.1128/jb.81.6.858-862.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFADDEN B. A., RAMANANDARAO G. ENZYMES OF THE TRICARBOXYLIC ACID CYCLE IN PSEUDOMONAS INDIGOFERA. Can J Microbiol. 1964 Jun;10:503–504. doi: 10.1139/m64-061. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Howes W. V. OXIDATIVE METABOLISM AND THE GLYOXYLATE CYCLE IN PSEUDOMONAS INDIGOFERA. J Bacteriol. 1962 Jul;84(1):72–76. doi: 10.1128/jb.84.1.72-76.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAI J. STUDIES ON ITACONATE METABOLISM. II. CITRAMALATE METABOLISM IN PSEUDOMONAS FLUORESCENS GROWN ON ITACONATE. J Biochem. 1963 Jul;54:34–40. doi: 10.1093/oxfordjournals.jbchem.a127743. [DOI] [PubMed] [Google Scholar]
- NAGAI J. Studies on itaconate metabolism. I. Itaconyl-Co-A synthesizing reaction in cell-free extracts of Pseudomonas fluorescens. J Biochem. 1963 Mar;53:181–187. doi: 10.1093/oxfordjournals.jbchem.a127678. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W. Enzymatic analysis of the pathways of glucose catabolism and gluconeogenesis in Pseudomonas citronellolis. Arch Microbiol. 1975 Mar 12;103(1):71–76. doi: 10.1007/BF00436332. [DOI] [PubMed] [Google Scholar]
- Patni N. J., Alexander J. K. Utilization of glucose by Clostridium thermocellum: presence of glucokinase and other glycolytic enzymes in cell extracts. J Bacteriol. 1971 Jan;105(1):220–225. doi: 10.1128/jb.105.1.220-225.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS O. C., RUTTER W. J. Preparation and properties of yeast aldolase. J Biol Chem. 1961 Dec;236:3177–3184. [PubMed] [Google Scholar]
- ROTHSTEIN M., MAYOH H. NEMATODE BIOCHEMISTRY. IV. ON ISOCITRATE LYASE IN CAENORHABDITIS BRIGGSAE. Arch Biochem Biophys. 1964 Oct;108:134–142. doi: 10.1016/0003-9861(64)90364-9. [DOI] [PubMed] [Google Scholar]
- Rao G. R., McFadden B. A. Isocitrate lyase from Pseudomonas indigofera. IV. Specificity and inhibition. Arch Biochem Biophys. 1965 Nov;112(2):294–303. doi: 10.1016/0003-9861(65)90049-4. [DOI] [PubMed] [Google Scholar]
- Roche T. E., Williams J. O., McFadden B. A. Effect of pH and buffer upon Km and inhibition by phosphoenolpyruvate of isocitrate lyase from Pseudomonas indigofera. Biochim Biophys Acta. 1970 Apr 22;206(1):193–195. doi: 10.1016/0005-2744(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Rosen S. M., Horecker B. L. Purification and properties of a specific fructose 1,6-diphosphatase from Candida utilis. Arch Biochem Biophys. 1965 Dec;112(3):411–420. doi: 10.1016/0003-9861(65)90073-1. [DOI] [PubMed] [Google Scholar]
- Rothstein M., Mayoh H. Nematode biochemistry. VII. Presence of isocitrate lyase in Panagrellus redivivus. Turbatrix aceti, and Rhabditis anomala. Comp Biochem Physiol. 1965 Dec;16(4):361–365. doi: 10.1016/0010-406x(65)90302-6. [DOI] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. Poly- -hydroxybutyrate biosynthesis and the regulation of glucose metabolism in Azotobacter beijerinckii. Biochem J. 1971 Nov;125(1):55–66. doi: 10.1042/bj1250055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stribling D., Perham R. N. Purification and characterization of two fructose diphosphate aldolases from Escherichia coli (Crookes' strain). Biochem J. 1973 Apr;131(4):833–841. doi: 10.1042/bj1310833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Williams J. O., Roche T. E., McFadden B. A. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry. 1971 Apr 13;10(8):1384–1390. doi: 10.1021/bi00784a017. [DOI] [PubMed] [Google Scholar]



