Abstract
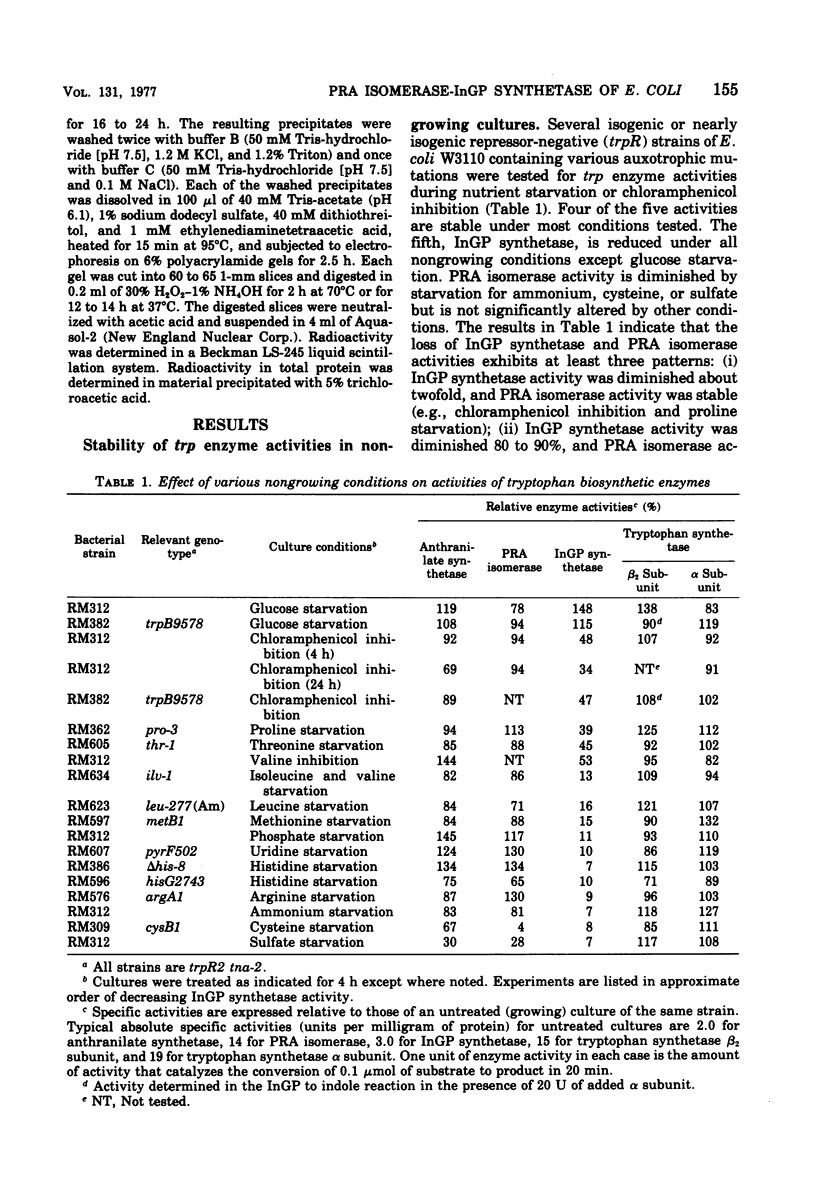
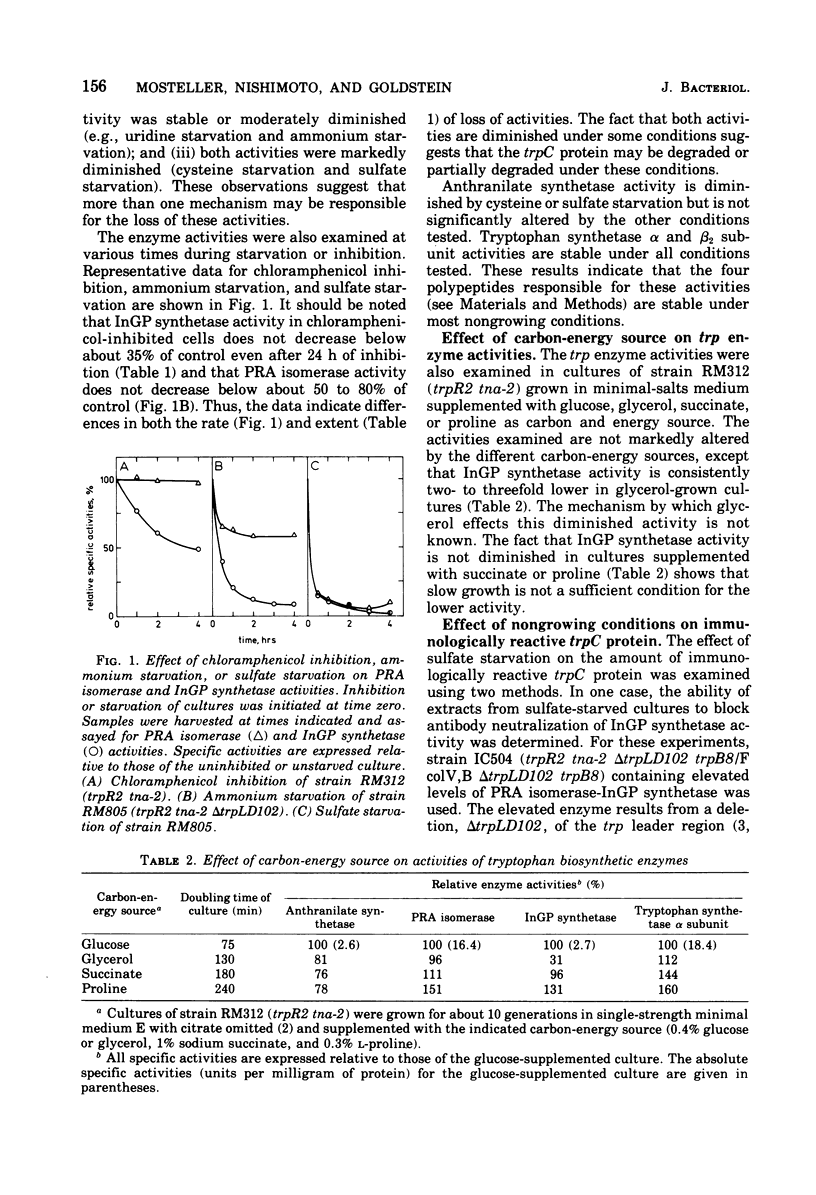
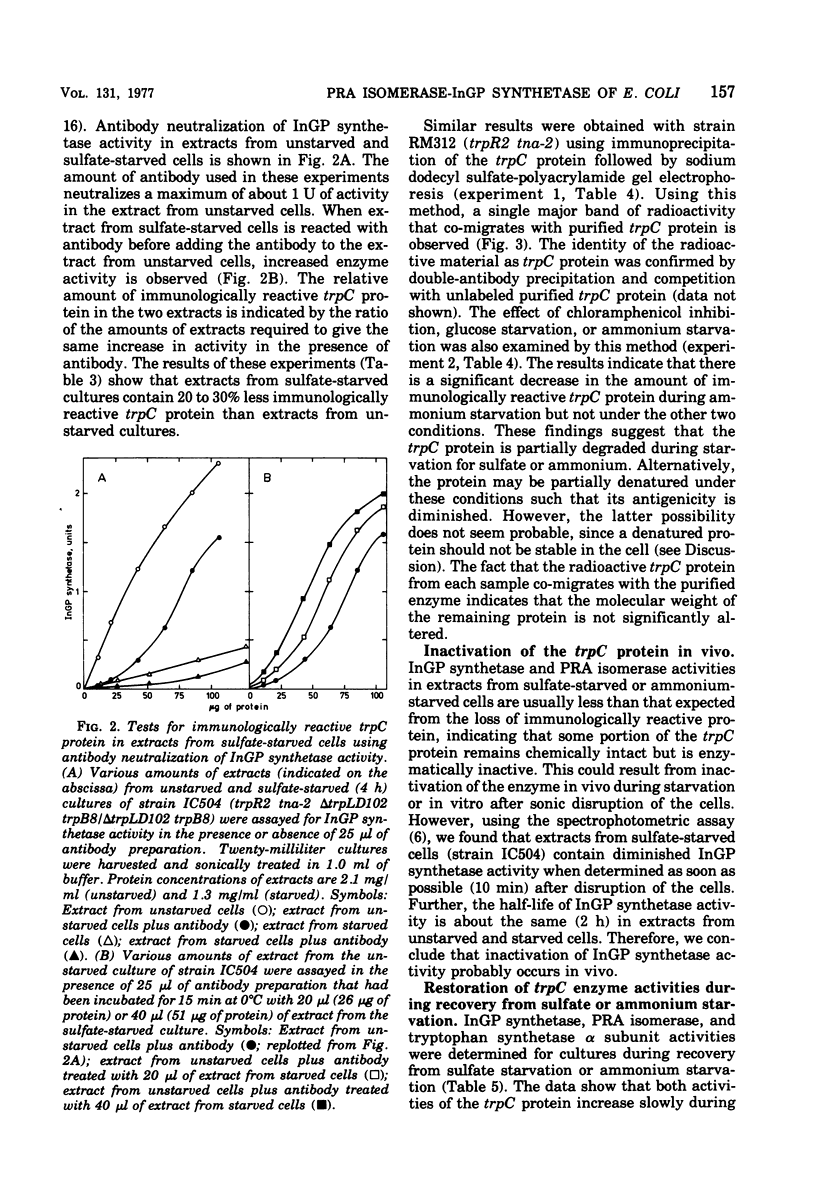
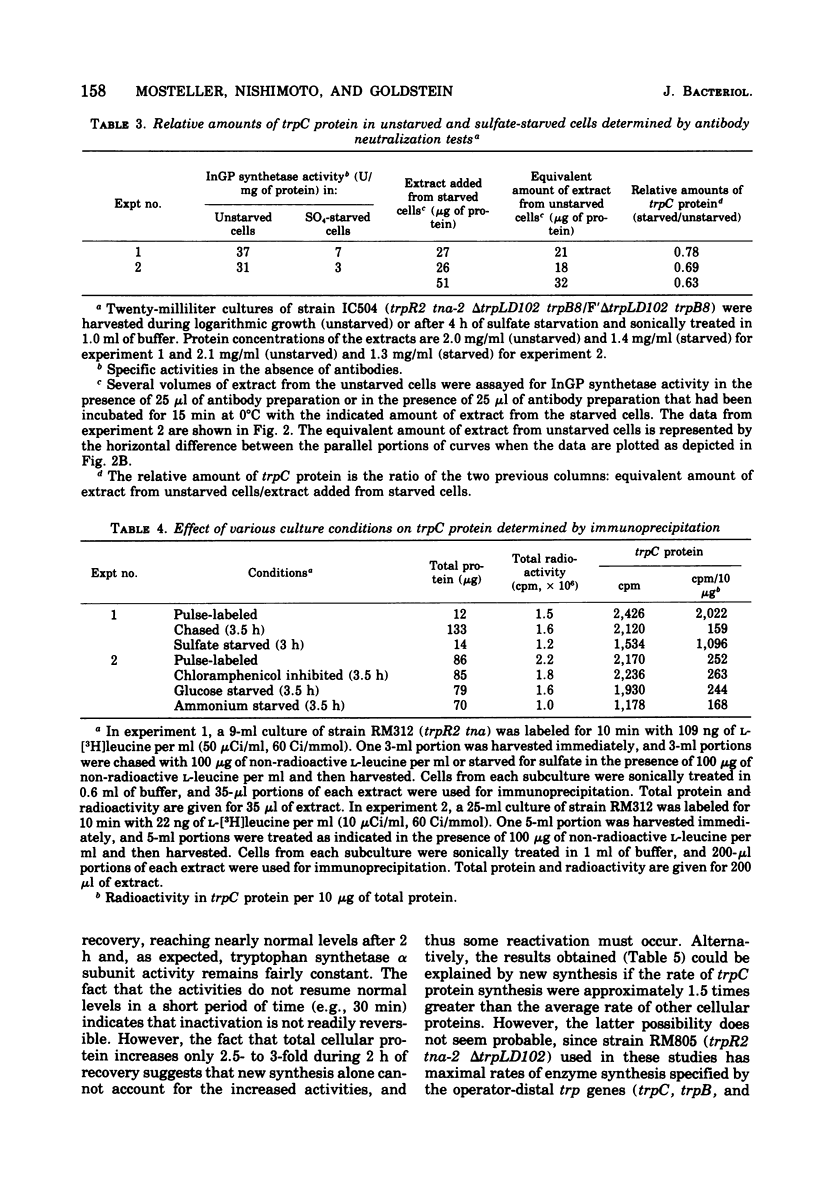
The stability of tryptophan biosynthetic enzyme activities was examined in cultures of repressor-negative (trpR) strains of Escherichia coli K-12 incubated under conditions of nutrient starvation of chloramphenicol inhibition. The results show that four of the five activities examined are stable under most nongrowing conditions, whereas one activity, indoleglycerol phosphate (InGP) synthetase, carried by the trpC protein, is unstable under most conditions tested. Phosphoribosylanthranilate (PRA) isomerase activity, which is also carried by the trpC protein, is unstable during starvation for ammonium, cysteine, or sulfate but is stable under other nongrowing conditions where InGP synthetase is not. InGP synthetase activity but not PRA isomerase activity is also diminished about twofold in cultures using glycerol as a carbon-energy source. These results indicate that one or both activities of the trpC protein is specifically inactivated under several culture conditions. Experiments with antibodies to the trpC protein show that sulfate-starved and ammonium-starved cultures contain 20 to 40% less immunologically reactive trpC protein than unstarved cultures. This indicates that the trpC protein is probably partially degraded under these conditions. During recovery from sulfate starvation or ammonium starvation, cultures slowly regain normal levels of InGP synthetase and PRA isomerase activities, suggesting that inactivation may be reversible.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger F. G., Herrmann K. M. Tryptophan synthetase alpha(5.7-S): novel molecular species formed within Escherichia coli. J Bacteriol. 1975 Nov;124(2):800–809. doi: 10.1128/jb.124.2.800-809.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Squires C., Yanofsky C. Transcription termination in vivo in the leader region of the tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):319–337. doi: 10.1016/0022-2836(76)90315-6. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. N-(5'-phosphoribosyl)anthranilate isomerase-indol-3-ylglycerol phosphate synthetase of tryptophan biosynthesis. Relationship between the two activities of the enzyme from Escherichia coli. Biochem J. 1970 Dec;120(4):699–707. doi: 10.1042/bj1200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- GIBSON F., YANOFSKY C. The partial purification and properties of indole-3-glycerol phosphate synthetase from Escherichia coli. Biochim Biophys Acta. 1960 Oct 7;43:489–500. doi: 10.1016/0006-3002(60)90471-6. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Correlation between rates of degradation of bacterial proteins in vivo and their sensitivity to proteases. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2640–2644. doi: 10.1073/pnas.69.9.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D. An upper limit to the protein content of the germinal substance of bacteriophage T2. Virology. 1955 May;1(1):108–127. doi: 10.1016/0042-6822(55)90009-x. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Thr region between the operator and first structural gene of the tryptophan operon of Escherichia coli may have a regulatory function. J Mol Biol. 1973 May 5;76(1):89–101. doi: 10.1016/0022-2836(73)90082-x. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li S. S., Hanlon J., Yanofsky C. Amino-terminal sequences of indoleglycerol phosphate synthetase of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1975 Aug;123(2):761–764. doi: 10.1128/jb.123.2.761-764.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- McQuade J. F., 3rd, Creighton T. E. Purification and comparison of the N-(5'-phosphoribosyl)anthranilic acid isomerase-indole-3-glycerol phosphate synthetase of tryptophan biosynthesis from three species of Enterobacteriaceae. Eur J Biochem. 1970 Oct;16(2):199–207. doi: 10.1111/j.1432-1033.1970.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Mandula B. B. Kinetics of derepression of the tryptophan operon of Escherichia coli and Salmonella typhimurium under different culture conditions. J Mol Biol. 1973 Nov 15;80(4):801–823. doi: 10.1016/0022-2836(73)90211-8. [DOI] [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. II. Strain differences in the degradation of protein and nucleic acid resulting from starvation. J Biol Chem. 1971 Nov 25;246(22):6956–6967. [PubMed] [Google Scholar]
- Niles E. G., Westhead E. W. The variable subunit structure of lysine-sensitive aspartylkinase from Escherichia coli TIR-8. Biochemistry. 1973 Apr 24;12(9):1715–1722. doi: 10.1021/bi00733a009. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Regulation of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Jul;115(1):107–116. doi: 10.1128/jb.115.1.107-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Steady-state measurement of the turnover of amino acid in the cellular proteins of growing Escherichia coli: existence of two kinetically distinct reactions. J Bacteriol. 1970 Jul;103(1):207–215. doi: 10.1128/jb.103.1.207-215.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Miller J. H., Weber K. In vivo degradation of mutant lac repressor. Nature. 1970 Dec 19;228(5277):1154–1156. doi: 10.1038/2281154a0. [DOI] [PubMed] [Google Scholar]
- Prouty W. F., Karnovsky M. J., Goldberg A. L. Degradation of abnormal proteins in Escherichia coli. Formation of protein inclusions in cells exposed to amino acid analogs. J Biol Chem. 1975 Feb 10;250(3):1112–1122. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELA M., GIVOL D., MOZES E. RESOLUTION OF RABBIT GAMMA-GLOBULIN INTO TWO FRACTIONS BY CHROMATOGRAPHY ON DIETHYLAMINOETHYL-SEPHADEX. Biochim Biophys Acta. 1963 Dec 13;78:649–657. doi: 10.1016/0006-3002(63)91031-x. [DOI] [PubMed] [Google Scholar]
- SHORTMAN K., LEHMAN I. R. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. VI. CHANGES IN ENZYME LEVELS IN RESPONSE TO ALTERATIONS IN PHYSIOLOGICAL STATE. J Biol Chem. 1964 Sep;239:2964–2974. [PubMed] [Google Scholar]
- Scheps R., Revel M. Deficiency in initiation factors of protein synthesis in stationary-phase Escherichia coli. Eur J Biochem. 1972 Sep 18;29(2):319–325. doi: 10.1111/j.1432-1033.1972.tb01991.x. [DOI] [PubMed] [Google Scholar]
- Smith O. H. Structure of the trpC cistron specifying indoleglycerol phosphate synthetase, and its localization in the tryptophan operon of Escherichia coli. Genetics. 1967 Sep;57(1):95–105. doi: 10.1093/genetics/57.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Villarejo M. R., Zabin I. Beta-galactosidase from termination and deletion mutant strains. J Bacteriol. 1974 Oct;120(1):466–474. doi: 10.1128/jb.120.1.466-474.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in non-growing cells of Escherichia coli. Biochem J. 1967 May;103(2):453–461. doi: 10.1042/bj1030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser D., Bhavsar P. Missense mutations in the lacZ gene that result in degradation of beta-galactosidase structural protein. J Bacteriol. 1976 Sep;127(3):1538–1542. doi: 10.1128/jb.127.3.1538-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



