Abstract
Metastasis is the ultimate life-threatening stage of cancer. The lack of accurate model systems thwarted studies of the metastatic cell’s basic biology. To follow continuously the succeeding stages of metastatic colony growth, we heritably labeled cells from the human lung adenocarcinoma cell line ANIP 973 with green fluorescent protein (GFP) by transfection with GFP cDNA. Labeled cells were then injected intravenously into nude mice, where, by 7 days, they formed brilliantly fluorescing metastatic colonies on mouse lung [Chishima, T., Miyagi, Y., Wang, X., Yang, M., Tan, Y., Shimada, H., Moossa, A. R. & Hoffman, R. M. (1997) Clin. Exp. Metastasis 15, 547–552]. The seeded lung tissue was then excised and incubated in the three-dimensional sponge-gel-matrix-supported histoculture that maintained the critical features of progressive in vivo tumor colonization while allowing continuous access for measurement and manipulation. Tumor progression was continuously visualized by GFP fluorescence in the same individual cultures over a 52-day period, during which the tumors spread throughout the lung. Histoculture tumor colonization was selective for lung cancer cells to grow on lung tissue, because no growth occurred on histocultured mouse liver tissue, which was also observed in vivo. The ability to support selective organ colonization in histoculture and visualize tumor progression by GFP fluorescence allows the in vitro study of the governing processes of metastasis [Kuo, T.-H., Kubota, T., Watanbe, M., Furukawa, T., Teramoto, T., Ishibiki, K., Kitajima, M., Moossa, A. R., Penman, S. & Hoffman, R. M. (1995) Proc. Natl. Acad. Sci. USA 92, 12085–12089]. The results presented here provide significant, new opportunities to understand and to develop treatments that prevent and possibly reverse metastasis.
Keywords: tumor colonization, lung cancer, host lung, sponge-gel histoculture, green fluorescent protein
Paget, more than 100 years ago, formulated his seed and soil hypothesis (i.e., the cells from a given tumor would “seed” only favorable “soil” offered by certain organs; ref. 1). He hypothesized that cancer cells must find a suitable “soil” in a target organ (i.e., one that supports colonization) for metastasis to occur. We recently reported that the ability of human colon cancer cells to colonize liver tissue governs whether a particular colon cancer will metastasize to the liver (2). In the model used in that study, human colon tumors were transplanted into the nude mouse colon as intact tissue blocks by surgical orthotopic implantation (i.e., to the corresponding organ). At the site of implantation in the mouse colon, all tumors were equally invasive locally into tissues and blood vessels. However, the metastatic behavior of implanted tumors closely depended on the metastatic behavior of the original human patient tumor. Only those tumors that originally metastasized to the liver in the donor patient did so in the mouse model. Furthermore, the cells of these metastasizing tumors could also colonize the liver directly after intrahepatic implantation. In contrast, cells from nonmetastatic colon tumors failed to engraft when implanted into liver tissue. Thus, the ability to colonize a distant organ is a critical aspect of metastatic spread in tumor progression. However, the colonization of distant tissues by micrometastasis formation and subsequent growth is still poorly understood due to lack of suitable experimental models.
In this report, we describe a new system for the study of the metastatic process in vitro. Until now, envisioning an in vitro experimental system for replicating metastatic colony establishment and progression was not possible. Three recent developments make the described system realizable. One is the technology that underlies surgical orthotopic implantation. Tumor cells will closely replicate their original behavior in a heterologous species if implanted onto the corresponding organ. The experiments described here used human lung cancer cells growing on mouse lung. Another important technique is an in vitro culture system that closely mimics in vivo conditions. To this end, we took advantage of collagen sponge-gel matrix histoculture introduced by Leighton (3) in the 1950s and further developed by us (4–12). Finally, we sought a rapid, nondestructive means for examining the cultured lung tissue so that continuous measurements could be made. This requirement was met by developing a line of human lung cancer cells expressing the jellyfish Aequorea victoria green fluorescent protein (GFP) (13–23). Metastatic colony growth was followed over a 52-day period in the three-dimensional sponge-gel-matrix-supported histoculture. These results provide an invaluable new tool for understanding the most important steps in tumor host-organ interaction, tumor progression, and metastasis. Because the metastatic colony expansion takes place in vitro, it offers a unique opportunity for developing agents for intervention.
MATERIALS AND METHODS
Human Lung Tumor Cell Line.
The human lung adenocarcinoma cell line (ANIP 973) was obtained from the Department of Molecular Biology, Harbin Medical University, Harbin, China.
GFP Expression Vector.
The dicistronic expression vector (pED-mtxr) was obtained from Genetics Institute (Cambridge, MA) (24). The expression vector containing the codon-optimized hGFP-S65T gene (21) was purchased from CLONTECH. To construct the hGFP-S65T-containing expression vector, phGFP-S65T was digested with HindIII to blunt the end. The hGFP entire coding region was then excised with XbaI. The pED-mtxr vector was digested with PstI, blunted at the end, and further digested with XbaI. The hGFP-S65T cDNA fragment was then unidirectionally subcloned into pED-mtxr. The resulting vector was used to transfect the human lung tumor cells with the GFP gene (22, 23).
Cell Culture, Transfection, and Subcloning.
ANIP 973 cells were cultured in RPMI 1640 medium (GIBCO) containing 10% fetal calf serum (FCS) (Gemini Biological Products, Calabasas, CA) and 2 mM l-glutamine. For transfection, near-confluent ANIP 973 cells were incubated with a precipitated mixture of LipofectAMINE Reagent (GIBCO) and saturating amounts of plasmid for 6 hr before being replenished with fresh medium (23).
ANIP 973 cells were harvested by trypsin/EDTA 48 hr posttransfection. The cells were subcultured at a ratio of 1:15 into selective medium that contained 50 nM methotrexate (MTX). Cells with stably integrated expression vector containing the GFP gene were selected by growing transiently transfected cells in the MTX-containing medium. Clones were isolated with cloning cylinders (Bel-Art Products) by trypsin/EDTA. The clones were amplified and transferred by conventional culture methods. Clone-26 was selected due to its high-intensity GFP fluorescence (23).
Animals and Experimental Metastases.
Six-week-old BALB/c nu/nu male mice were injected intravenously with 5 × 106 Clone-26 cells. Cells were first harvested by trypsinization and washed three times with cold serum-containing medium and then kept on ice. Cells were then injected in a total volume of 0.5 ml within 40 min of harvesting. The nude mice were sacrificed 7 days after tumor cell injection.
Tumor Progression in Histoculture.
Whole lung tissues seeded with GFP Clone-26 cells were aseptically removed from the nude mice. The lung tissues were divided into pieces of approximately 2–3 mm in diameter and then placed on prehydrated collagen sponge gels (Upjohn). The gels were floated in 24-well plates at the air–water interface in RPMI 1640 medium containing 20% FCS (Gemini Biological Products), 2 mM l-glutamine, and penicillin. The histocultures were incubated at 37°C in a humidified atmosphere containing 95% air and 5% CO2. The lung tumor colony growth in the histocultured host lung tissue was repeatedly observed in the same cultures with fluorescence photomicroscopy of the GFP expression at days 6, 14, 24, and 52 of histoculture.
To determine specificity of colonization, GFP Clone-26 tissue fragments from subcutaneously grown tumors were seeded into small wells made in the mouse lung and liver tissue that had already been placed in histoculture.
Microscopy.
Fluorescence microscopy was carried out using a Nikon microscope equipped with a xenon lamp power supply and a Leica stereo fluorescence microscope model LZ12 equipped with a mercury lamp power supply. Both microscopes had a GFP filter set (Chroma Technology, Brattleboro, VT).
RESULTS AND DISCUSSION
Transfection of GFP Expression Vector into Human Lung Cancer Cells.
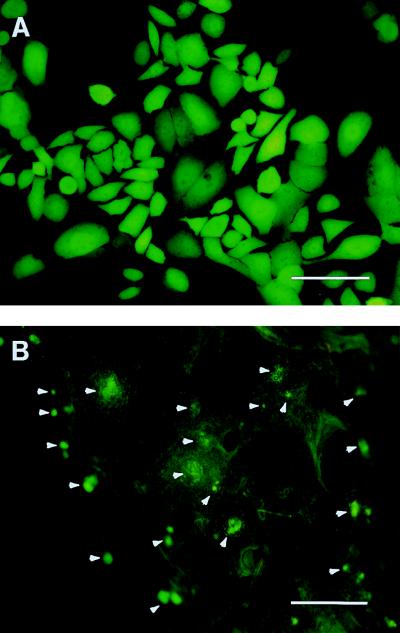
The crucial first step in using GFP for tracking the fate of metastasizing cells is to stably label an appropriate line of cancer cells. We chose mouse lung as the host tissue, because its morphology is especially suited to early detection of metastatic colonies and it lends itself to subsequent in vitro incubation. The cell selected for GFP labeling was the human lung cancer line ANIP 973. When injected intravenously, these cells seed numerous colonies on the mouse lung, where they mimic the original growth on human lung tissue (23). The transfected cells were selected in MTX and can grow in levels of up to 50 nM. The MTX-resistant ANIP 973 cells developed a striking increase in GFP fluorescence after subcloning, compared with transiently transfected cells. A subclone that had the strongest GFP expression was isolated and termed ANIP 973 Clone-26 (Clone-26) (Fig. 1A) (23).
Figure 1.
Mouse lung seeding in vivo by GFP human lung tumor cells. (A) GFP-transfected cells selected in 50 nm MTX. Approximately 1.0 × 107 cells were injected into the tail vein of nude mice. (B) The excised mouse lung 7 days after cell injection. Numerous fluorescent small colonies can be visualized on the nude mouse lung, with a size of up to approximately 150 microns. Portions of this lung were then explanted into collagen-sponge-gel-matrix-supported histoculture (Bar = 500 μm.)
Lung Colonization by the GFP-Transfected Lung Tumor Cells in Nude Mice.
The route of cells metastasizing to lung tissue was mimicked by injecting 1.0 × 107 Clone-26 cells into the tail vein of nude mice. Seven days after injection, numerous micrometastatic colonies were visualized in the excised whole lung tissue by GFP fluorescence (Fig. 1B). These colonies, which remained strongly fluorescent, had developed up to approximately 150 μm in diameter.
Lung Colony Growth by the GFP-Transfected Lung Tumor Cells in Histoculture.
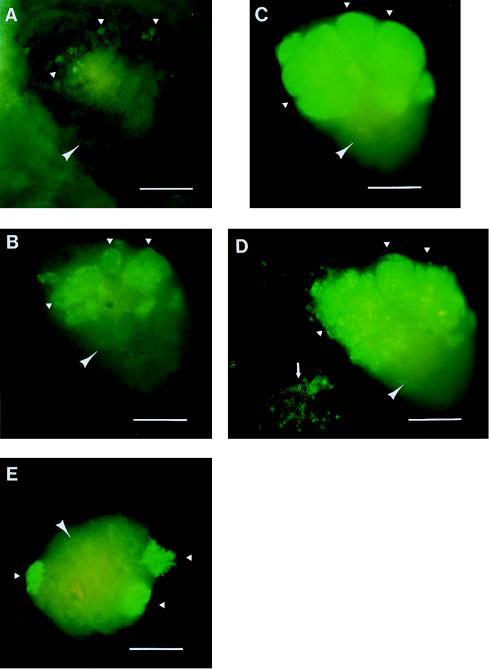
Clone-26-seeded mouse lungs were removed from the mice and then histocultured on collagen sponge gels. Tumor colonies grew and spread rapidly in the lung tissue over time in histoculture. We could visualize continuously the progressive colonization of normal lung tissue by the lung tumor cells in individual cultures. Fig. 2 A–D is an example of tumor colony growth on a single fragment of lung tissue. After 6 days in histoculture, the tumor colonies were still classifiable as microcolonies (Fig. 2A). However, by day 14, very extensive growth of the colonies had occurred with three different areas of visible GFP-labeled malignant cells (Fig. 2B). By day 24 of histoculture, the tumor colonies had grown significantly, reaching sizes of 750 μm in diameter and involving approximately one-half of the histocultured mouse lung (Fig. 2C). By 52 days of histoculture, tumor cells had involved the lung even more extensively and appeared to form multiple layers and histologically suggestive structures on the histocultured lung (Fig. 2D). Also, by day 52, GFP-expressing satellite tumor colonies formed in the sponge gel distant from the primary colonies in the lung tissue (Fig. 2D). Fig. 2E represents a parallel histoculture at day 24, in which the colonizing lung tumor is spectacularly visible on the normal mouse lung.
Figure 2.
Mouse lung colonization by GFP human lung tumor cells in histoculture. (A) Growth of the tumor cells seeded in the mouse lung after 6 days of histoculture. The tumor colonies still remain as microcolonies with an average size of approximately 90 μm. By day 14, however, the tumor colonies had grown very significantly and occupied a significant fraction of the lung, with the largest individual colony being approximately 400 μm across its largest diameter (B). By day 24, very brightly fluorescing tumor colonies had grown to occupy approximately one-half of the histocultured lung, with sizes up to 750 μm (C). A parallel, 24-day histocultured lung had brightly fluorescing tumor colonies that invaded the supporting sponge-gel matrix as well as the lung itself (E). (D) At 52 days of histoculture, the tumor colonies have grown more on the histocultured mouse lung, with perhaps a secondary layer of colonies forming a structure on top of the first layer of colonies. The colonies were differentiating rather than expanding at this point. Satellite colonies by then had grown into the supporting sponge-gel matrix (Bar = 500 μm.)
Specificity of Host Tissue for Successful Colonization.
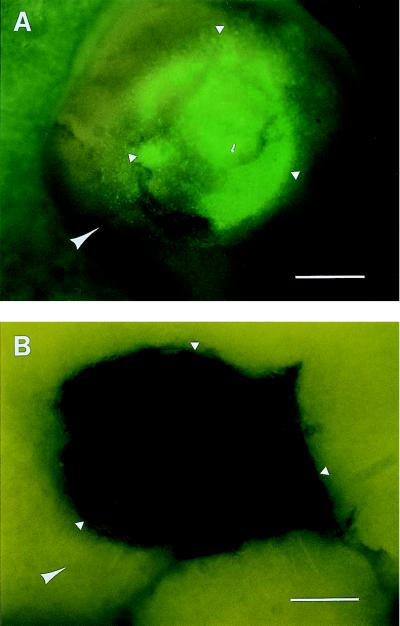
To determine the specificity of tumor host-tissue colonization, GFP-expressing ANIP 973 tissue fragments were placed in small wells made in mouse lung tissue and in liver tissue that had already been placed in histoculture. The results were striking in their contrast. When the lung tumor fragments were put on the mouse lung, extensive growth and GFP fluorescence were seen at day 29 (Fig. 3A). However, when the lung tumor fragments were put on the liver, no tumor could be detected. Even by day 29, only a dark hole was apparent in the well of the liver in which the tumor had been implanted.
Figure 3.
Specificity of host-organ colonization in histoculture. (A) Fragments of GFP-fluorescent ANIP 973 colonies were placed on histocultured mouse lung. At day 29, the tissue was vigorously growing and fluorescing. (B) The same as A except that the GFP-fluorescing ANIP 973 fragments were placed on normal mouse liver in histoculture. By day 29, no live ANIP could be detected. (Bar = 400 μM.)
We thus have shown the replication and visualization of selective organ colonization and colony growth in vitro using histoculture of host organs and a GFP-transfected human lung adenocarcinoma. It has previously proven difficult to study the mechanisms of metastasis and tumor progression, because micrometastases and microcolonization usually develop in internal organs in vivo and can be visualized by current imaging procedures only at relatively late stages. Current in vivo imaging procedures do not permit continual direct observation of the ongoing process of tumor colonization and progression over the time course of the disease.
Chambers et al. (25, 27) and others (26) visualized the extravasation and initial seeding steps in tumor metastasis using nonheritably fluorescent-labeled tumor cells in vivo. Margolis et al. (28) visualized the migration of nonheritably fluorescently labeled lung tumor cells in host mouse lung in three-dimensional histoculture. Both the Chambers (25, 26) and Margolis (28) studies were not able to visualize the long-term growth progression of metastatic colonies in host organs because the cells were not heritably labeled with fluorescence. In contrast, the GFP gene transfectants described here have high levels of GFP expression that are heritable and stable, and the tumor cells can be visualized throughout the tumor progression. Reporter genes such as lacZ have been used to trace metastases in vivo (29), but this type of gene requires tissue processing for visualization and therefore does not allow the continuous, nondestructive, repetitive monitoring of tumor progression that is possible with GFP as a reporter.
In the present study, GFP gene-transfected human lung cancer cells seeded in vivo on the mouse lung were used to visualize the colonization of mouse lung starting from microcolonies and progressing to major colonies. These occupied the majority of the host lung tissues after 52 days in histoculture. Tumor colonies grew to more than 750 μm in diameter on the host lung tissue in vitro, eventually occupying approximately one-half of the organ. The GFP-transfected tumor cells also invaded and colonized the supporting sponge-gel matrix. The colonization in histoculture is also selective for the lung as a very supportive soil, as opposed to the liver. This mimics seeding in vivo when the lung tumor cells injected in the tail vein primarily colonize mouse lung tissue. The lung selectivity is also seen after orthotopic implantation into mouse lung followed by metastasis to the contralateral lung (23).
The tumor host-organ chimeric histoculture system developed in the present study with GFP fluorescing tumor cells has significantly advanced the ability to understand and treat human metastatic cancer.
ABBREVIATIONS
- GFP
green fluorescent protein
- MTX
methotrexate
References
- 1.Paget S. Lancet. 1889;i:571–573. [Google Scholar]
- 2.Kuo T, Kubota T, Watanbe M, Furukawa T, Teramoto T, Ishibiki K, Kitajima M, Moossa A R, Penman S, Hoffman R M. Proc Natl Acad Sci USA. 1995;92:12085–12089. doi: 10.1073/pnas.92.26.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leighton J. Cancer Res. 1957;17:929–941. [PubMed] [Google Scholar]
- 4.Freeman A, Hoffman R M. Proc Natl Acad Sci USA. 1986;83:2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vescio R A, Redfern C H, Nelson T J, Ugoretz S, Stern P H, Hoffman R M. Proc Natl Acad Sci USA. 1987;84:5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman R M, Monosov A Z, Connors K M, Herrera H, Price J H. Proc Natl Acad Sci USA. 1989;86:2013–2017. doi: 10.1073/pnas.86.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman R M. Cancer Cells. 1991;3:86–92. [PubMed] [Google Scholar]
- 8.Hoffman R M. Stem Cells (Dayton) 1993;11:105–111. doi: 10.1002/stem.5530110205. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman R M. Crit Rev Oncol. 1993;15:99–111. doi: 10.1016/1040-8428(93)90050-e. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa T, Kubota T, Hoffman R M. Clin Cancer Res. 1995;1:305–311. [PubMed] [Google Scholar]
- 11.Kubota T, Sasano N, Abe O, Nakao I, Kawamura E, Saito T, Endo M, Kimura K, Demura H, Sasano H, Nagura H, Ogawa N, Hoffman R M. Clin Cancer Res. 1995;1:1537–1543. [PubMed] [Google Scholar]
- 12.Geller J, Partido C, Sionit L, Youngkin T, Espanol M, Tan Y, Hoffman R M. Prostate. 1997;31:250–254. doi: 10.1002/(sici)1097-0045(19970601)31:4<250::aid-pros6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 14.Cheng L, Fu J, Tsukamoto A, Hawley R G. Nat Biotechnol. 1996;14:606–609. doi: 10.1038/nbt0596-606. [DOI] [PubMed] [Google Scholar]
- 15.Prasher D C, Eckenrode V K, Ward W W, Prendergast F G, Cormier M J. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 16.Yang F, Miss L G, Phillips G N., Jr Nat Biotechnol. 1996;14:1252–1256. [Google Scholar]
- 17.Cody C W, Prasher D C, Welstler V M, Prendergast F G, Ward W W. Biochemistry. 1993;32:1212–1218. doi: 10.1021/bi00056a003. [DOI] [PubMed] [Google Scholar]
- 18.Heim R, Cubitt A B, Tsien R Y. Nature (London) 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 19.Delagrave S, Hawtin R E, Silva C M, Yang M M, Youvan D C. Bio/Technology. 1995;13:151–154. doi: 10.1038/nbt0295-151. [DOI] [PubMed] [Google Scholar]
- 20.Cramer A, Whitehorn E A, Tate E, Stemmer W P C. Nat Biotechnol. 1996;14:315–319. doi: 10.1038/nbt0396-315. [DOI] [PubMed] [Google Scholar]
- 21.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzycka N. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chishima T, Miyagi Y, Wang X, Yamaoka H, Shimada H, Moossa A R, Hoffman R M. Cancer Res. 1997;57:2042–2047. [PubMed] [Google Scholar]
- 23.Chishima T, Miyagi Y, Wang X, Yang M, Tan Y, Shimada H, Moossa A R, Hoffman R M. Clin Exp Metastasis. 1997;15:547–552. doi: 10.1023/a:1018431128179. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman R J, Davies M V, Wasley L C, Michnick D. Nucleic Acids Res. 1991;19:4485–4490. doi: 10.1093/nar/19.16.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers A F, MacDonald I C, Schmidt E E, Koop S, Morris V L, Khokha R, Groom A C. Cancer Metastasis Rev. 1995;14:279–301. doi: 10.1007/BF00690599. [DOI] [PubMed] [Google Scholar]
- 26.Khokha R, Groom A C. Cancer Metastasis Rev. 1995;14:279–301. doi: 10.1007/BF00690599. [DOI] [PubMed] [Google Scholar]
- 27.Koop S, MacDonald I C, Luzzi K, Schmidt E E, Morris V L, Grattan M, Khokha R, Chambers A, Groom A C. Cancer Res. 1995;55:2520–2523. [PubMed] [Google Scholar]
- 28.Margolis L B, Glushakova S E, Baibakov B A, Collin C, Zimmerberg J. In Vitro Cell Dev Biol. 1995;31:211–226. doi: 10.1007/BF02639437. [DOI] [PubMed] [Google Scholar]
- 29.Lin W-C, Pretlow T P, Pretlow T C, Culp L A. J Natl Cancer Inst. 1990;82:1497–1503. doi: 10.1093/jnci/82.18.1497. [DOI] [PubMed] [Google Scholar]