Abstract
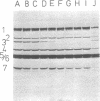
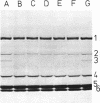
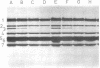
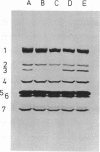
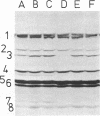
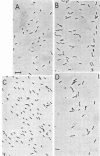
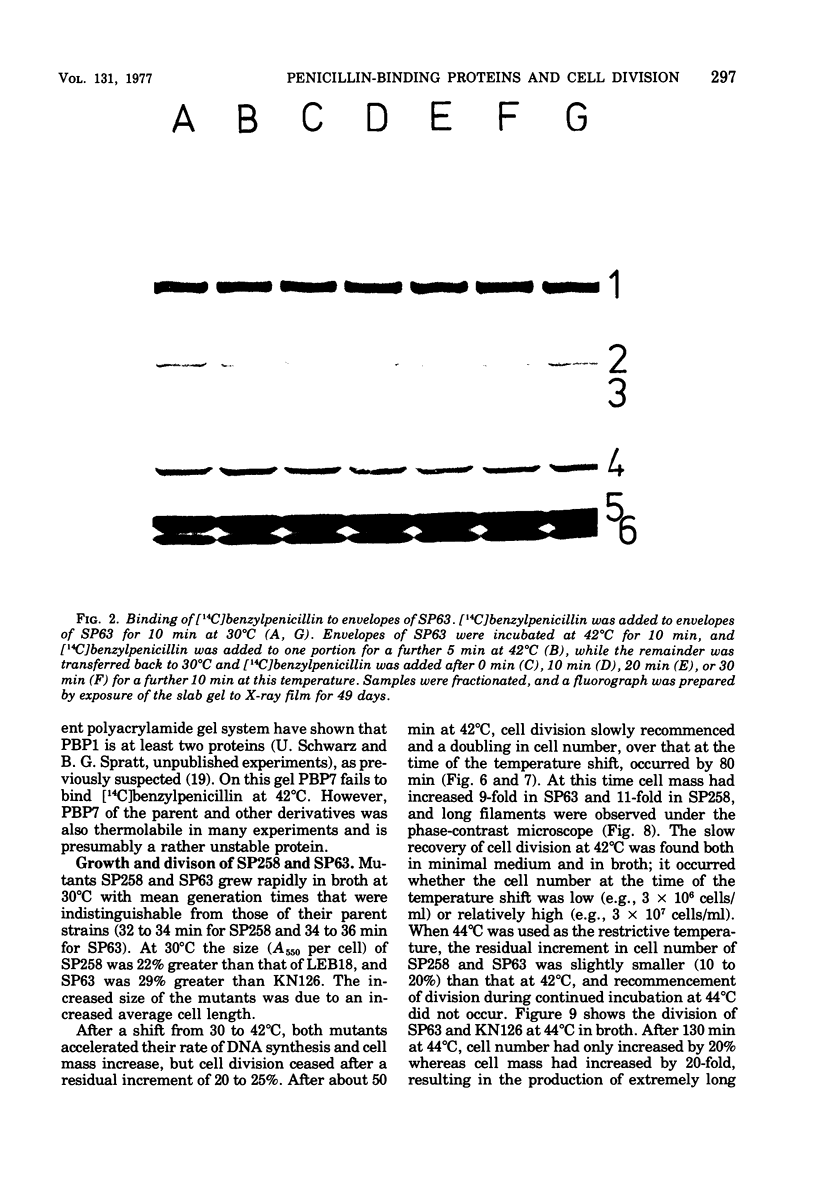
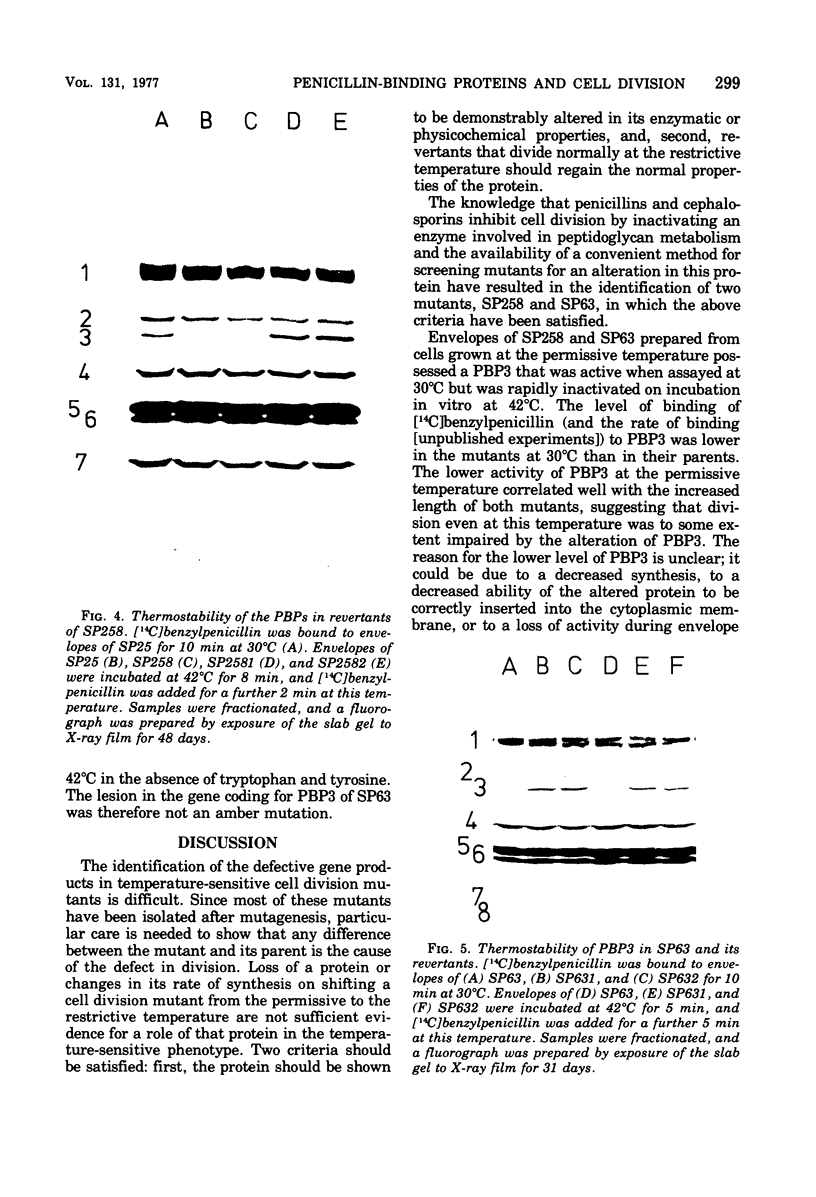
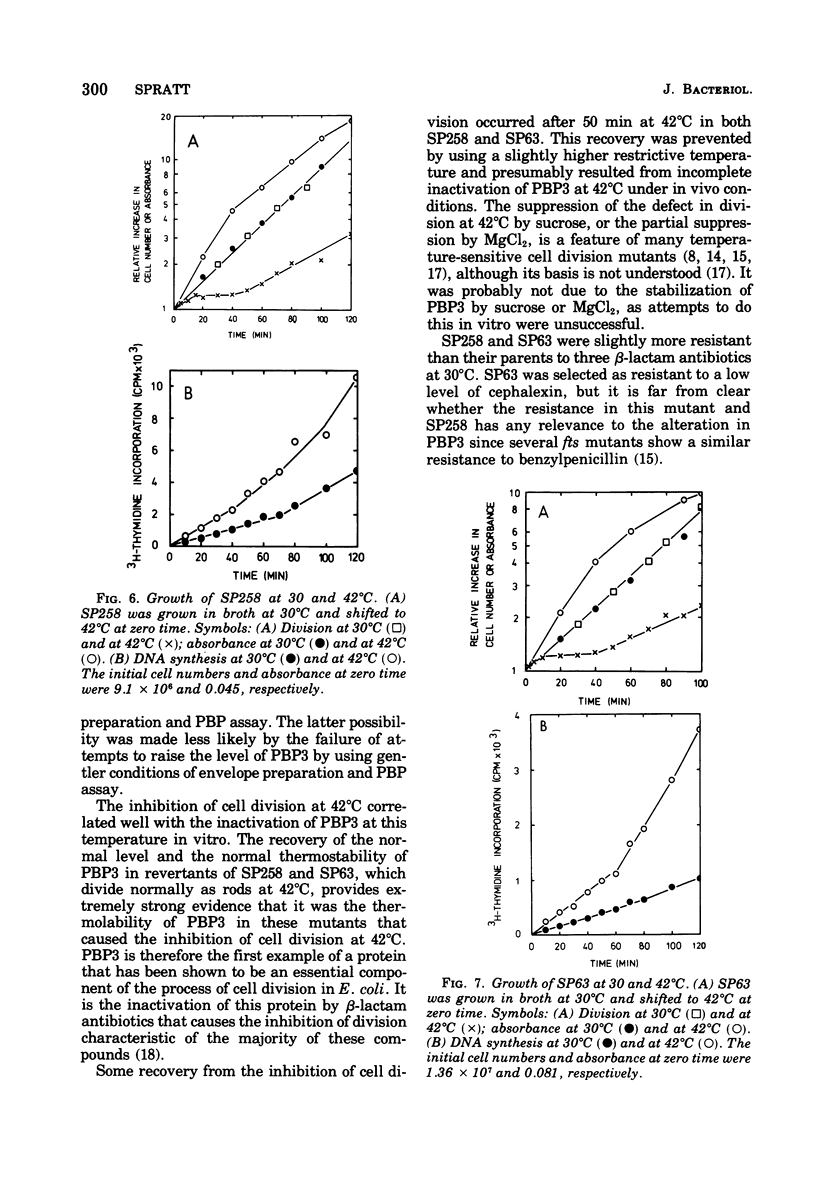
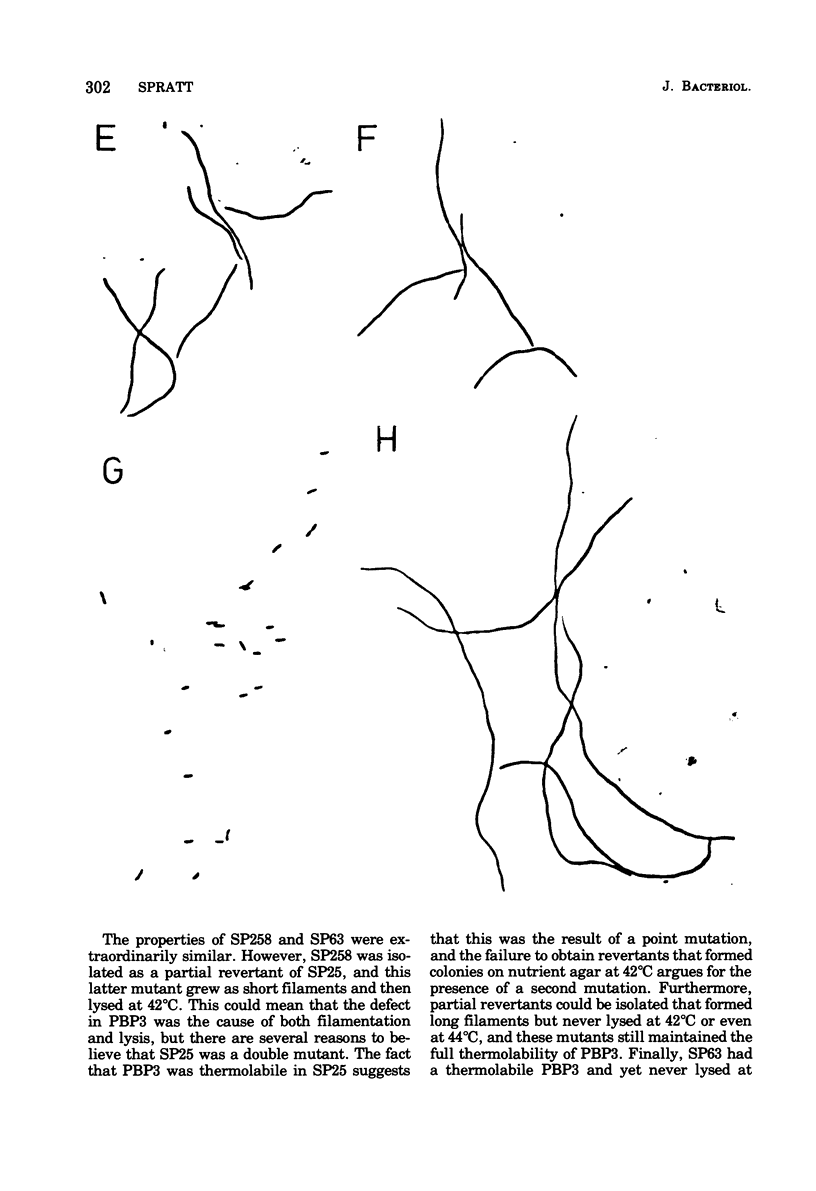
The thermostability of the penicillin-binding proteins (PBPs) of 31 temperature-sensitive cell division mutants of Escherichia coli has been examined. Two independent cell division mutants have been found that have highly thermolabile PBP3. Binding of [14C]benzylpenicillin to PBP3 (measured in envelopes prepared from cells grown at the permissive temperature) was about 30% of the normal level at 30°C, and the ability to bind [14C]benzylpenicillin was rapidly lost on incubation at 42°C. The other PBPs were normal in both mutants. At 30°C both mutants were slightly longer than their parents and on shifting to 42°C they ceased dividing, but cell mass and deoxyribonucleic acid synthesis continued and long filaments were formed. At 42°C division slowly recommenced, but at 44°C this did not occur. The inhibition of division at 42°C was suppressed by 0.35 M sucrose, and in one of the mutants it was partially suppressed by 10 mM MgCl2. PBP3 was not stabilized in vitro at 42°C by these concentrations of sucrose or MgCl2. Revertants that grew as normal rods at 42°C regained both the normal level and the normal thermostability of PBP3. The results provide extremely strong evidence that the inactivation of PBP3 at 42°C in the mutants is the cause of the inhibition of cell division at this temperature and identify PBP3 as an essential component of the process of cell division in E. coli. It is the inactivation of this protein by penicillins and cephalosporins that results in the inhibition of division characteristic of low concentrations of many of these antibiotics.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix D. E., Helmstetter C. E. Coupling between chromosome completion and cell division in Escherichia coli. J Bacteriol. 1973 Sep;115(3):786–795. doi: 10.1128/jb.115.3.786-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Darby V. Genetical and physiological studies on a thermosensitive mutant of Escherichia coli defective in cell division. J Gen Microbiol. 1976 Jan;92(1):156–166. doi: 10.1099/00221287-92-1-156. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Nagata T., Horiuchi T. Isolation and characterization of a temperature-sensitive amber suppressor mutant of Escherichia coli K12. Mol Gen Genet. 1973;123(1):77–88. doi: 10.1007/BF00282991. [DOI] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Onken A., Messer W. Cell division in Escherichia coli septation during synchronous growth. Mol Gen Genet. 1973 Dec 31;127(4):349–358. doi: 10.1007/BF00267105. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Comparison of the binding properties of two 6 beta-amidinopenicillanic acid derivatives that differ in their physiological effects on Escherichia coli. Antimicrob Agents Chemother. 1977 Jan;11(1):161–166. doi: 10.1128/aac.11.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. Physiological and genetical studies on a mutant of Salmonella typhimurium which is temperature-sensitive for DNA synthesis. Mol Gen Genet. 1972;114(1):35–49. doi: 10.1007/BF00268745. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Woldringh C. L. Morphological analysis of nuclear separation and cell division during the life cycle of Escherichia coli. J Bacteriol. 1976 Jan;125(1):248–257. doi: 10.1128/jb.125.1.248-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]