Abstract
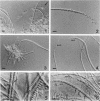
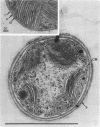
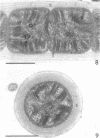
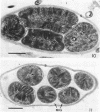
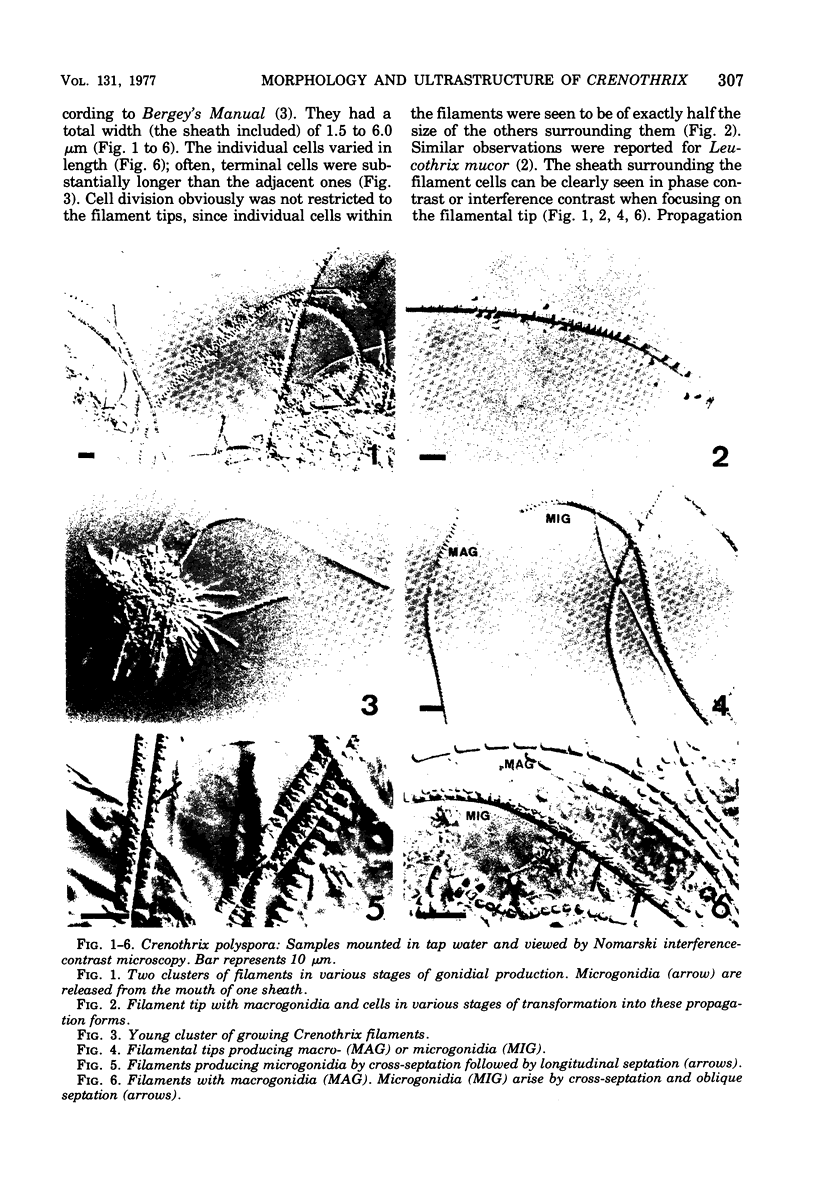
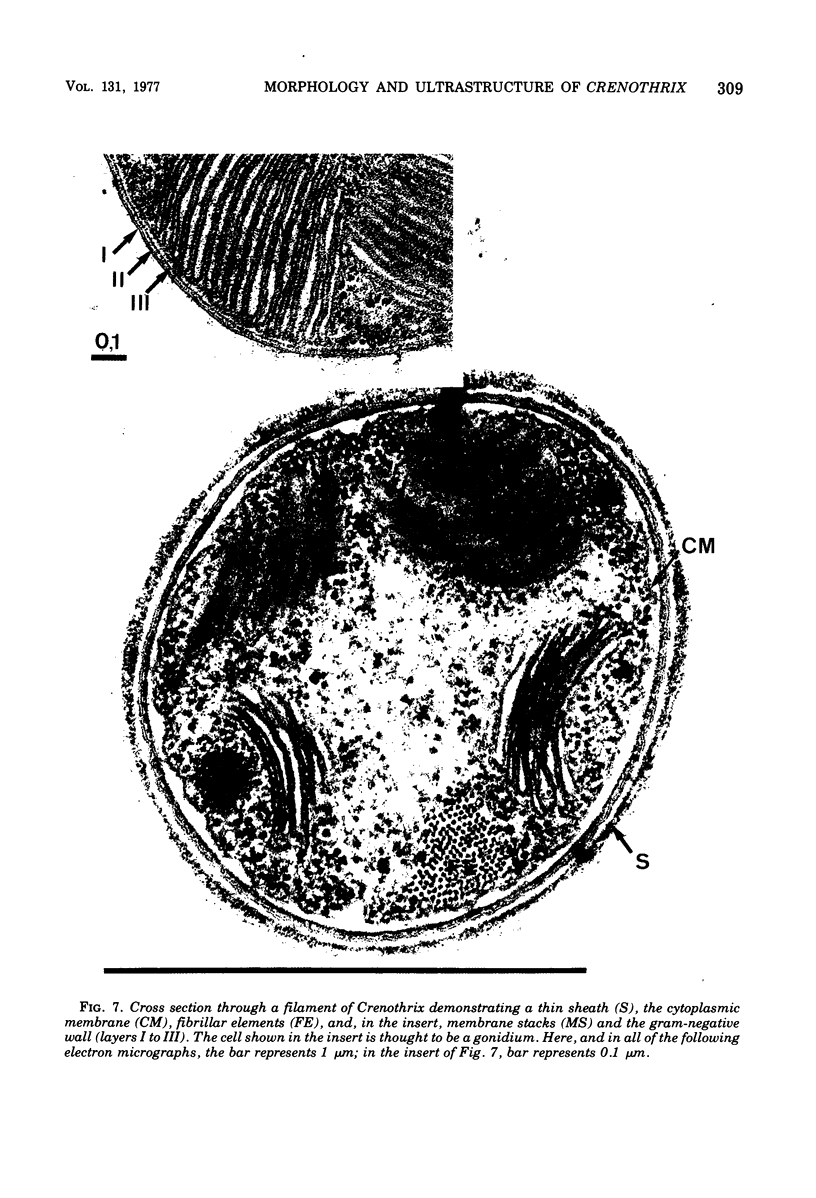
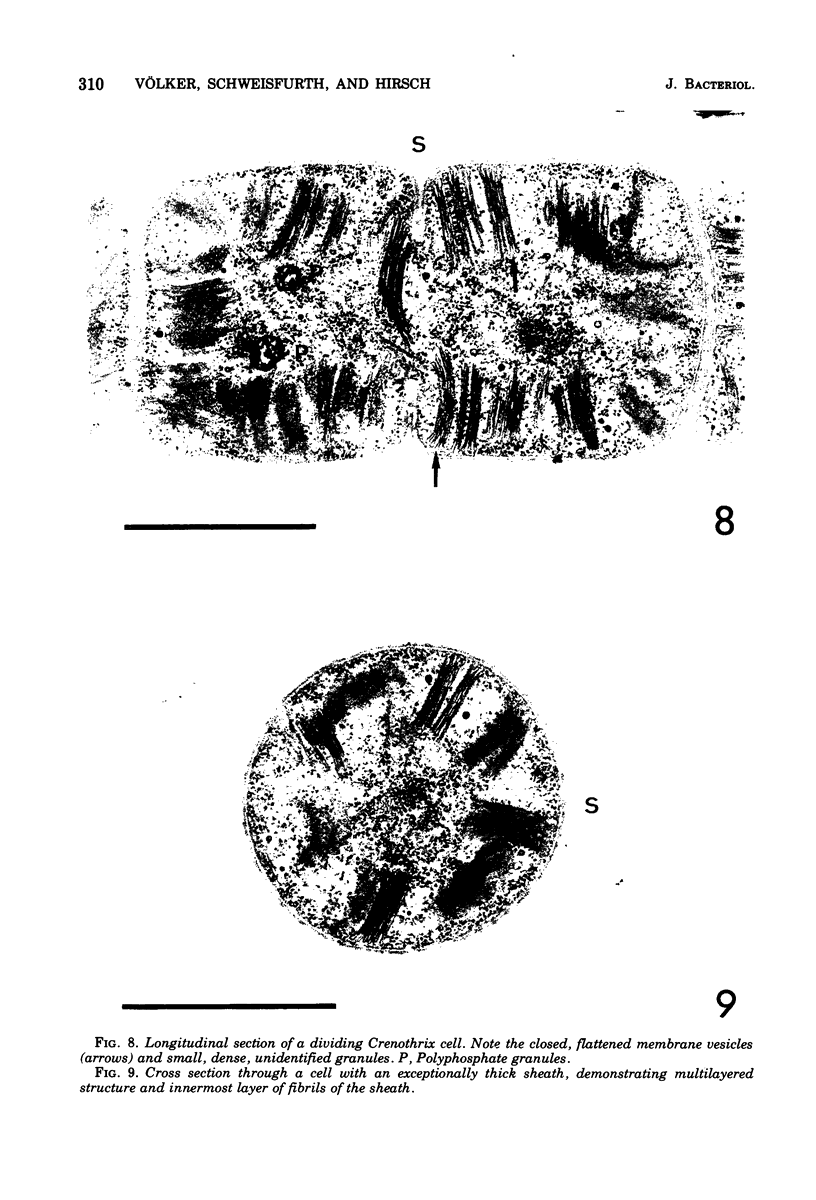
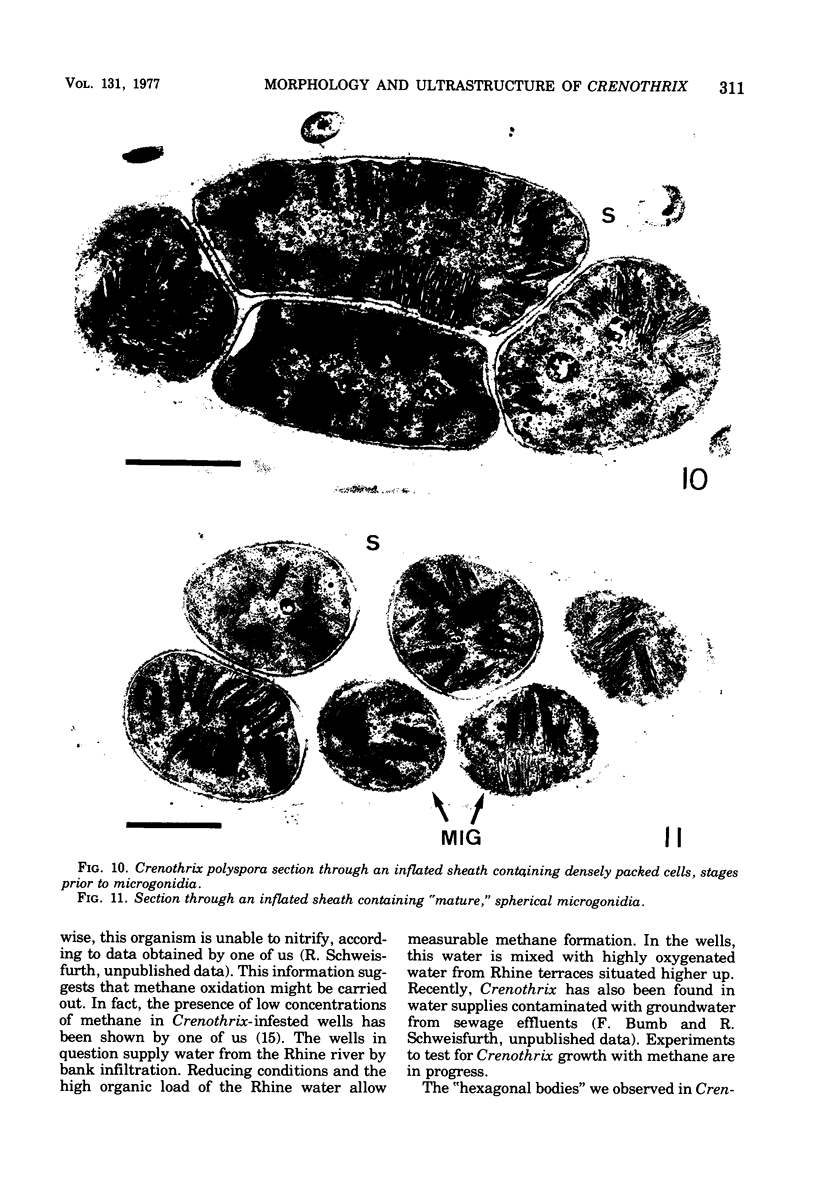
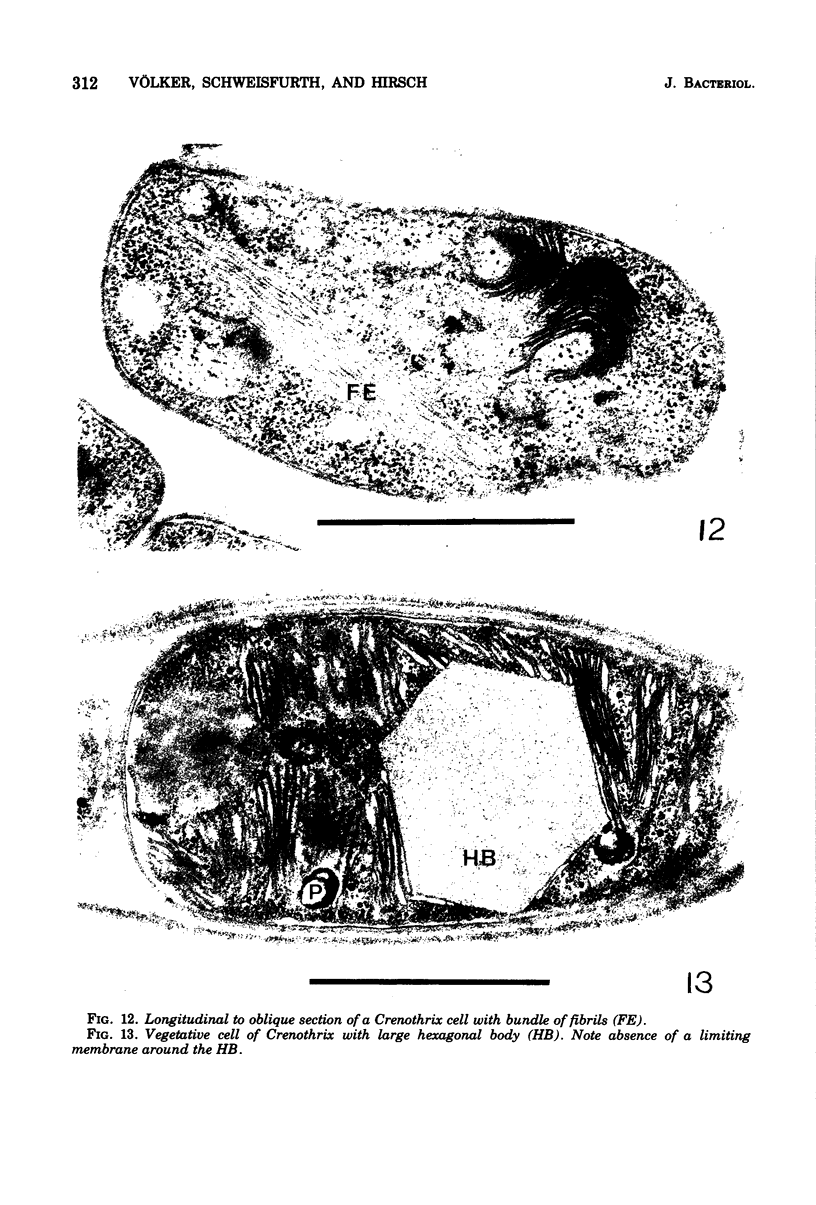
Naturally grown cell material of Crenothrix polyspora from the well of a waterworks was studied by means of phase-contrast and Nomarski interference microscopy as well as by transmission electron microscopy. The material consisted of clusters of sheathed filaments up to 2 cm long. Propagation forms observed were nonmotile, spherical cells that arose by simple ("macrogonidia") or multiple ("microgonidia") septation of the filamental tips. Ultrastructural analysis revealed Crenothrix to be procaryotic and gram negative, with several layers of sheath material surrounding the filaments. On thin sections, individual cells had elaborate membrane systems in the form of lamellar stacks. They resembled thylakoids of photosynthetic bacteria. Spectrophotometric analysis gave no indication of photosynthetic pigments. The cells also contained large hexagonal bodies, rod-shaped fibrillar elements, and polyphosphate granules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D. Mode of filamentous growth of Leucothrix mucor in pure culture and in nature, as studied by tritiated thymidine autoradiography. J Bacteriol. 1967 Mar;93(3):985–990. doi: 10.1128/jb.93.3.985-990.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Ultrastructure of blue-green algae. J Bacteriol. 1969 Mar;97(3):1486–1493. doi: 10.1128/jb.97.3.1486-1493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae T. H., McCurdy H. D. Ultrastructural studies of Chondromyces crocatus vegetative cells. Can J Microbiol. 1975 Nov;21(11):1815–1826. doi: 10.1139/m75-264. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Wildman R. B., Bowen C. C. Phycobilisomes in blue-green algae. J Bacteriol. 1974 Feb;117(2):866–881. doi: 10.1128/jb.117.2.866-881.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Physiology and cytological chemistry blue-green algae. Bacteriol Rev. 1973 Mar;37(1):32–101. doi: 10.1128/br.37.1.32-101.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]