Abstract
Divalent cations are thought essential for motile function of leukocytes in general, and for the function of critical adhesion molecules in particular. In the current study, under direct microscopic observation with concomitant time-lapse video recording, we examined the effects of 10 mM EDTA on locomotion of human blood polymorphonuclear leukocytes (PMN). In very thin slide preparations, EDTA did not impair either random locomotion or chemotaxis; motile behavior appeared to benefit from the close approximation of slide and coverslip (“chimneying”). In preparations twice as thick, PMN in EDTA first exhibited active deformability with little or no displacement, then rounded up and became motionless. However, on creation of a chemotactic gradient, the same cells were able to orient and make their way to the target, often, however, losing momentarily their purchase on the substrate. In either of these preparations without EDTA, specific antibodies to β2 integrins did not prevent random locomotion or chemotaxis, even when we added antibodies to β1 and αvβ3 integrins and to integrin-associated protein, and none of these antibodies added anything to the effects of EDTA. In the more turbulent environment of even more media, effects of anti-β2 integrins became evident: PMN still could locomote but adhered to substrate largely by their uropods and by uropod-associated filaments. We relate these findings to the reported independence from integrins of PMN in certain experimental and disease states. Moreover, we suggest that PMN locomotion in close quarters is not only integrin-independent, but independent of external divalent cations as well.
Divalent cations are thought to be essential for motile function of leukocytes in general, and specifically for the function of adhesion molecules critical to a number of their motile activities (1, 2). In examining effects of concentrations of EDTA in the anticoagulant range (≥1.8 mM) for other reasons, we noted that polymorphonuclear leukocytes (PMN) in warmed sealed slide preparations were not impaired in either random locomotion or chemotaxis. In the current study we have pushed the concentration of EDTA to a point (10 mM) where [Ca2+]i or [Mg2+]i in the medium would be <nM, with similar results.
In the slide preparations, a chemotactic gradient is created by the targeted destruction of one or a few erythrocytes resulting from a brief laser flash (3). We examine the behavior of PMN under direct microscopic observation with concomitant time-lapse video recording. Hence, we can follow directly and continuously the orientation and trajectory of PMN before, during, and after establishment of a chemotactic gradient (4).
In a sense, the results with EDTA make additional work on specific adhesion molecules redundant, as the function of all of them is thought to be divalent-cation-dependent. However, an examination of relevant leukocyte integrins through the use of mAbs was of interest so that their effects in this system, or lack of effects, might be placed in context with a variety of reported effects in other systems. Also implicit in the leukocyte work are its implications for the requirements for locomotion of other cell types, for example, in embryonic development or in cancer.
METHODS
Chemicals.
EDTA in a stock solution of 200 mM was prepared by dissolution of its free acid (Sigma) in PBS without Ca2+ or Mg2+, and titration to pH 7 with sodium hydroxide. Human serum albumin (HSA; Centre National de Transfusion Sanguine, Paris) was dissolved in PBS for each usage at a final concentration of 2%.
Antibodies.
mAb against β2 (CD18) integrins (R15.7, lot 061094) was the kind gift of C. Wayne Smith (Baylor, Houston), who has found it to inhibit neutrophil adhesion to glass coated with keyhole limpet hemocyanin [a membrane attack complex (MAC)-1[CD11b/CD18]-dependent function], adhesion of neutrophils to human hepatocytes [a function dependent on both lymphocyte function-associated antigen (LFA)-1 [CD11a/CD18] and MAC-1, and adherence-dependent hydrogen peroxide production by neutrophils (MAC-1-dependent) (C. Wayne Smith, personal communication).
mAb against β1 (CD29; P5D2) and αvβ3 (CD51/CD61, vitronectin receptor; 7G2) integrins, and against integrin-associated protein (IAP; CD47; B6H12), an Ig family member, were the kind gift of Eric Brown (Washington University, St. Louis). This particular αvβ3 mAb also binds the β3 integrin termed the leukocyte response integrin (E. Brown, personal communication), and IAP interacts with both of them.
Neutrophils.
PMN were contained in the buffy coat of fresh heparinized blood from human donors, which was allowed to sediment in tubes at an angle of 45° at room temperature for 1 hr. These leukocytes, along with a small number of erythrocytes, were suspended in autologous plasma (or occasionally in HSA buffer, as noted), with or without 10 mM EDTA, and with or without antibodies, each at ≥10 μg/ml, alone or in combination, for 10 min at room temperature. The cells were concentrated by centrifugation in the same liquor. A drop of this suspension sufficient to wet the area was deposited between a clean glass slide and glass coverslip, and the preparation was sealed with paraffin and removed to the warmed (37°C) stage of a Zeiss phase-contrast photomicroscope (objective, ×40), connected via a Hamamatsu Microscope Video Camera C2400 (Hamamatsu Photonics, Hamamatsu City, Japan) to a Panasonic Time Lapse Video Recorder AG6720 (Matsushita Electric Industrial, Osaka, Japan).
Chemotaxis.
A microscopic field containing an erythrocyte, or adjacent ones, and PMN was chosen. Erythrocytes were destroyed by a ruby laser microbeam (wavelength, 694.3 min; duration of flash, 0.5 msec) focused backward through the optics of the microscope to a diameter of ≈5 μm, creating a chemotactic gradient that lasts for 20 to 45 min, and the PMN were observed and recorded in time-lapse video microscopy (×16 real time) (5).
RESULTS
Effects of EDTA: Standard (Thin) Preparations.
In standard slide preparations, neither random locomotion nor chemotaxis of PMN were inhibited by the presence of 10 mM EDTA (Fig. 1). This was so whether the cellular elements had been suspended in autologous plasma or in HSA buffer. Monocytes and eosinophils also locomote randomly in EDTA, but neither cell type responds chemotactically in this system.
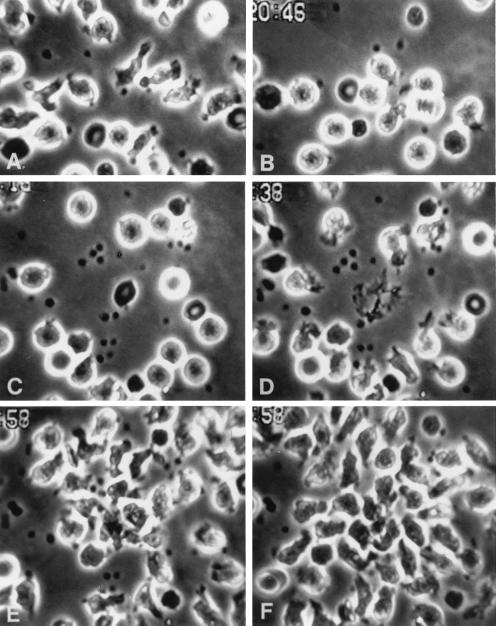
Figure 1.

EDTA (10 mM) in standard (thin) preparations does not inhibit either random locomotion or chemotaxis of PMN. Slides were prepared as described in Methods. (A) PMN are locomoting freely on substrate. (B) Thirty-nine seconds after laser destruction of erythrocytes, PMN are orienting toward the resulting target (t), addressing the chemotactic gradient that has been created; several protopods are marked by arrows. The spirals represent moving erythrocytes. (C) About 8 min later, PMN are surrounding the target (approx. ×650).
In considering this curious result, we wondered whether it could be attributed to our traditional way of preparing slides. For maximal images we use only enough suspending medium to wet the entire coverslip, formerly estimated by eye. We now measured that amount which, for the coverslip currently in use (22 mm × 32 mm) was ≈4 μl, and calculated the height of the suspending medium between slide and coverslip at 5.7 μm. This is an average value; it is visually obvious that cells in some regions of the preparation are more compressed than are those in other areas. However, given that the mean diameter of a PMN is ≈8 μm (ref. 6; personal observations), the cells can be said to be operating in close quarters between slide and coverslip, i.e., generally in close apposition to both, and we thought they might be using both surfaces for locomotion (“chimneying”). To see how they would react in a less physically constrained environment, we doubled the volume of suspending medium (and therefore its average height). Henceforth, we refer to these new preparations as thick and to the standard ones as thin.
Effects of EDTA: Thick Preparations.
When the average height of the suspending medium was increased to 11.4 μm, locomotion of control PMN, both random and directed, was like that in thin preparations. In particular, cells adhered well to substrate, resisting the occasional fluid currents that best are appreciated through movements of the floating erythrocytes, and which occur more commonly on warming thick preparations than thin ones.
In contrast, cells in EDTA behaved quite differently from those in thin preparations. The PMN did engage in active deformations while in suspension or when first attached (Fig. 2A), but with little or no displacement on substrate. After several minutes of attachment they became round and largely motionless (Fig. 2 B and C). However, given a chemotactic stimulus, the same PMN became oriented and made their way toward the source of the gradient (Fig. 2 E and F), but not without some difficulty. These PMN had less purchase on the substrate than controls, as evidenced by less lateral thickness of the migrating cells, brief losses of adhesion visualized as slipping and sliding, and a susceptibility to being dislodged by fluid currents. Reestablishment of purchase was evident from the sudden cessation of drift of a dislodged cell. It is of interest that orientation to the chemotactic gradient was maintained by PMN while briefly dislodged. Hence, EDTA impaired adherence but not orientation, and orientation in the gradient did not collapse when adherence failed.
Figure 2.
EDTA (10 mM) in thick preparations inhibits random locomotion but not chemotaxis of PMN. Slides were prepared as described in Results. (A) PMN often exhibit deformations while in suspension or when first attached, but with little or no displacement on substrate, then (B) round up and become largely motionless. In a different field (C), several such cells are seen before laser destruction of the central erythrocyte. On chemotactic stimulation (seen here 49 sec after the laser flash), the same cells orient (D) and make their way to the target (E), seen here about 3 min after the laser flash, often, however, with slipping and sliding, indicating poor purchase on substrate. During these periods of drift, they generally maintain their orientation toward the target. By 5 min after the flash (F), considerable numbers of PMN have arrived from outside the field (approx. ×580).
Effects of β2-Integrin mAb: Thin and Thick Preparations.
In thin preparations with 10 μg/ml β2-integrin mAb [saturation/blocking conditions of this antibody for PMN being 5–10 μg/ml (C. W. Smith, personal communication)] the cells appeared essentially normal, and neither random locomotion nor chemotaxis were inhibited, even when the preincubation was extended to 1 ½ hr. Unlike the case with EDTA, this lack of effect was obtained in thick preparations.
In considering this second curious result, we increased the concentration of mAb from 10 to 70 μg/ml or more (Fig. 3), used HSA buffer as suspending medium rather than the usual autologous plasma, and obtained a fresh shipment of mAb which, like the first, was frozen until use (we later showed that leukocytes stained appropriately with this antibody on FACS analysis, as they did with antibody to other β integrins, below). Despite these maneuvers, the cells continued to behave normally. When EDTA was added to cells preincubated with mAb, the effects were those of EDTA alone.
Figure 3.
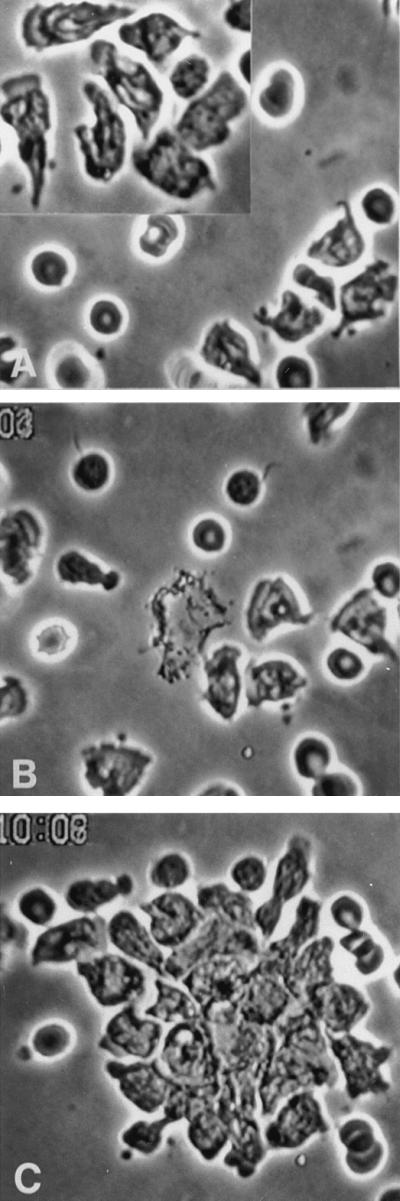
Antibody to β2 (CD18) integrins (70 μg/ml) in either thin or thick preparations does not inhibit either random locomotion or chemotaxis of PMN. Thin preparations are shown. (A) PMN are locomoting freely on substrate and did so even when the concentration of antibody was increased to 200 μg/ml (Inset). Note also the two motile monocytes at lower right of the inset. (B) Forty-one seconds after the laser flash, PMN are orienting toward the central target. (C) About 10 min later, PMN are surrounding the target. Results were the same when we added mAb to β1 and αvβ3 integrins and to IAP, and none of these antibodies added anything to the effects of EDTA alone (approx. ×520).
Although β2 integrins are the major integrin contributors to leukocyte motility and function, we then also used mAb to other β integrin subgroups expressed on PMN, β1, and β3, to heterodimers of which adhesive mechanisms have been ascribed, as well as IAP, which interacts with the relevant β3 integrins (7, 8).
Effects of Several PMN β-Integrin mAb.
There was no effect on random locomotion or chemotaxis of mAb to β1 and αvβ3 integrins and to IAP, each 10 μg/ml, whether used alone or all together (and together with β2 mAb), and whether in thin preparations or thick ones. The only difference from controls was that preparations that included IAP mAb, alone or in combination with other mAbs, contained agglutinated packs of erythrocytes (which also have IAP on their surfaces). Again, when EDTA was added to the preincubated cells, the effects were those of EDTA alone.
At this point the paradox was that β-integrin mAbs—and especially β2-integrin mAb, which neutralizes the most studied and apparently most important adhesive proteins experimentally and clinically (LFA-1, MAC-1, and p150/95)—had no apparent effect on random or directed locomotion. We now considered that the thick preparation, with an average calculated height of the suspending medium of 11.4 μm, still provided a fairly quiet environment, and, because PMN can secrete fresh β2 integrins (9, 10), perhaps on the occluded underside of the PMN, this might partially explain the difference between mAb and EDTA in thick preparations. We therefore increased the average height of the media to between 14 and 23 μm (most often 17–20 μm) in what we call very thick preparations.
Effects of β2-Integrin mAb: Very Thick Preparations.
In the more turbulent environment of very thick preparations, effects of β2-integrin mAb were at last evident. When control very thick preparations were warmed, the round, floating cells became progressively asymmetric as they began to adhere and locomote. Once settled, their entire undersurface appeared to adhere as they moved about either randomly or in a chemotactic gradient. In contrast, with 10 μg/ml β2-integrin mAb, PMN still could assume asymmetric shapes, but they adhered to substrate primarily by their uropods or by uropod-associated filaments, which impeded or sometimes prevented translocation (Fig. 4 A and B). In the example shown, PMN were able to pull free after a chemotactic stimulus (Fig. 4 C and D). Uropods of PMN also tended to adhere to each other, and sometimes to lymphocytes or monocytes (Fig. 4 A and B). In contrast, in thin preparations treated with mAb, although uropod-associated filaments were sometimes seen, especially with time, they did not impede random locomotion or chemotaxis.
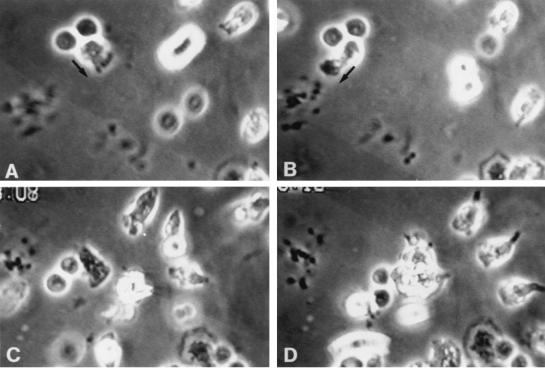
Figure 4.
Antibody to β2 (CD18) integrins (10 μg/ml) in very thick preparations causes PMN to adhere by their uropods, or by uropod-associated filaments, to substrate or to other cells. Slides were prepared as described in Results. Points of reference are a clump of platelets at 9 o’clock and a partially seen monocyte at 7 o’clock. (A) A PMN, oriented toward lower right (arrow), has two lymphocytes in tow and is stuck to the substrate by its uropod. (B) Seventy seconds later it randomly has reoriented toward lower left, but remains anchored to substrate, as do several other PMN in the field. (C) After creation of a chemotactic gradient, the index PMN has pulled itself free (as did others), and now, about 3 min later, nears the target (center), the lymphocytes still in tow. (D) About 2 min later, the initial PMN have merged with the target. (Right) Three new PMN have entered the field; note their prominent posterior filaments (approx. ×580).
DISCUSSION
In our standard, thin, sealed slide preparations of PMN in plasma or in HSA buffer, 10 mM EDTA did not impair either random locomotion or chemotaxis (Fig. 1). Appreciation of the locomotive abilities of PMN in the presence of EDTA appears due to the way in which the slide preparations customarily have been fashioned. They have three attributes relevant to this discussion: (i) cells are in close approximation to both slide and coverslip, (ii) the resulting chemotactic gradient concentrates itself into what approaches a single plane, maximizing the effect of what may be vanishingly small amounts of the exuding chemoattractant, and (iii) the cells are presented to best advantage for videomicroscopy (this having been the proximal reason for making them that way). In these preparations, eosinophils and monocytes also are able to locomote randomly in EDTA; however, they do not respond chemotactically in this system, even without EDTA.
Chimneying.
The first of these attributes bears special attention. Although small drops of suspended cells traditionally were planted on the slide by eye, we began to measure amounts that produced visually excellent (somewhat compressed) preparations, and, using the dimensions of the coverslip, calculated the average height of the fluid beneath it at 5.7 μm. As the average diameter of a PMN is 8 μm, the cells are in close quarters, and it was here that we found neither random locomotion nor chemotaxis to be impaired by EDTA. We suspect that the moving PMN are benefiting from both surfaces, as does a rock climber mounting a narrow cleft or chimney, and we refer to locomotion in these circumstances as chimneying.
Learning to Swim, But Not Swimming.
When we doubled the height of the fluid, the other side of the chimney was out of reach, and PMN in EDTA were seen to regress from a state of active deformability with little or no displacement, to rounded immobility (Fig. 2 A and B). However, this state of affairs ended when the cells were presented with a chemotactic stimulus (Fig. 2 C-F). Now they oriented and moved toward the source of the gradient, but with a much weaker than normal purchase on substrate. Although they maintained their orientation toward the target, in the videotapes they often were seen to slip and slide in their progress, then get an apparent “toe-hold” (sudden stopping of drift) and continue. We began to think of this as “learning to swim,” as it recalls a child’s first efforts at swimming, pointing in the right direction but only making progress when a foot touches bottom. It appears to represent a convergence, when adherence is impaired, of the ability of PMN to attain (here, to maintain) structural asymmetry in suspension on the one hand, and to execute directed surface locomotion on the other. It is doubtful from the videotapes that PMN ever properly learn to swim free, i.e., to make way in suspension; the propulsive force appears to depend upon their interaction with substrate. Thus, in thick preparations, EDTA prevented random locomotion and impaired adhesion, but did not affect orientation in a chemotactic gradient or directed locomotion per se.
Modeling Three Dimensions with Only Two.
The effect on PMN locomotion of antibodies against the common subunit of β2 integrins (including, for PMN, anti-LFA-1 [CD11a/CD18], anti-MAC-1 [CD11b/CD18], and anti-p150/95 [CD11c/CD18]) varies with the system used to study them. In careful assessments of unstimulated and chemoattractant-stimulated cells (11, 12), such antibodies effectively blocked locomotion under agarose, referred to as two-dimensional, but had little effect on three-dimensional movement through cellulose filters (Boyden chambers) or through collagen gel matrices. Moreover, PMN from LFA-1/MAC-1/p150,95-deficient patients, i.e., those with leukocyte adhesion deficiency 1, which have profoundly impaired movement on plane surfaces, moved normally in collagen gels (12). This work supported earlier evidence that locomotion is adhesion-dependent on plane surfaces, but largely adhesion-independent in three-dimensional matrices (13, 14). Locomotion in the latter situation was thought to result from the penetration of a narrow gap by a pseudopod, and its expansion distally to create an anchor whose purchase then would be used to bring along the rest of the cell (13–15). Such findings were consistent with the view that egress of PMN from the vascular space is adherence-dependent, but indicated that movement through extravascular tissues may not be (12).
Based on the current work we would amend the earlier formulations only in reference to dimensionality. We saw excellent random locomotion and chemotaxis of PMN treated with 10 μg/ml anti-β2 integrins, or even with much higher concentrations (Fig. 3), in PMN moving in two dimensions. The difference is likely in the physically close quarters in which they moved, especially in the standard thin preparations, in which they could chimney. Chimneying, then, would seem to provide a simple means of studying locomotion in two dimensions, while permitting force generation in three.
As for the thick preparations, a circumscribed suspending medium, whose height averages 11.4 μm, remains a relatively still pond, in which PMN that are continually up-regulating adhesion proteins from intracellular pools to the occluded underside of the the PMN (9, 10) may thwart antibody access sufficiently to allow random locomotion and chemotaxis to proceed, as in fact they did even when we added mAbs to other PMN integrins (β1, αvβ3) or integrin-associated protein, to which adhesive functions are ascribed (7, 8). Only in the more turbulent setting of very thick sealed preparations (average height of suspending medium generally 17–20 μm) did we see functional effects of β2 integrin mAb, and we saw them with the lowest concentration used (10 μg/ml; Fig. 4). There were both random and directed locomotion, but by PMN adhering primarily by their uropods or by uropod-associated filaments, to substrate, as well as to each other and sometimes to other cells (lymphocytes, monocytes). We suspect that this picture represents β2 integrins that are being secreted on the sequestered underside of locomoting PMN, out of harm’s (antibody’s) way, and are swept to the uropod of the moving cells, by which they then adhere to substrate (16) or sometimes complex with mAb and antigen from lymphocytes (largely via LFA-1; Fig. 4) or from other PMN or monocytes (via all three β2 integrins). It will be of interest to test the PMN of one of the rare patients with severe leukocyte adhesion deficiency 1 in this system, because they will have no functional β2 integrins to up-regulate.
In thin preparations of PMN treated with β2 integrin mAb, the cells were (perforce) flatter, and uropod-associated filaments either were not visible or did not appear to impede locomotion. For random locomotion, we suspect that potential impedance is overcome by the increased propulsive force that chimneying is likely to support; for directed locomotion, there is in addition a more powerful chemotactic stimulus in thin preparations, as its emanation from the source approaches a plane. However, we cannot rule out some degree of increase in up-regulation of integrins in the more turbulent environment of PMN in very thick preparations, a teleologically attractive possibility, i.e., integrins on demand, that will bear further scrutiny.
Other Integrin-Independent Locomotion in Vivo.
Our results may relate to apparent non-β2 integrin-dependent egress of PMN from the circulation that occurs, for example, in leukocyte adhesion deficiency 1 patients who develop pneumonia (17), as well as in some experimental pneumonias in mice given anti-β2 integrins (18) or lacking ligands for β2 integrins (19). Leukocyte sequestration and transmigration in the lung appear to occur primarily in capillaries (20) rather than in postcapillary venules. Although the current data cannot speak to precise molecular mechanisms of sequestration and transmigration into tissue, capillary diameters (mean 5.78 μm; ref. 21) are comparable to the height of the media in thin preparations, and hence locomotive behavior in them may be independent not only of integrins, but of any mechanism requiring external divalent cations on the part of PMN.
In summary, we conclude that whether in plasma or HSA buffer PMN locomotion in close quarters is independent not only of leukocyte integrins, but of external divalent cations as well. In a less physically constrained environment, when external divalent cations are unavailable, random locomotion ceases and adherence is impaired, but orientation and directed locomotion are preserved. When adherence fails, orientation persists; locomotion appears not to. PMN locomotion in close quarters provides a simple two-dimensional model for analysis of a type of non-integrin-dependent movement of PMN that has been thought to be restricted to three-dimensional matrices.
Acknowledgments
We are ever grateful to the late Professor Marcel Bessis, who made this work possible, to Drs. C. Wayne Smith and Eric Brown for mAb and advice, to Dr. Ruth Montgomery for critical review of the manuscript, to Dr. Joan Smallwood for useful discussions, and to Dr. Brian Smith for FACS analysis. This work was supported in part by the U.S. Public Health Service (Grants AR-10493, TE-02039, and AI-30548) and by the Fondation de France, Paris (Grants 921741 and 931732). S.E.M. was a Senior Fellow of the Fogarty International Center.
ABBREVIATIONS
- PMN
polymorphonuclear leukocytes
- HSA
human serum albumin
- LFA
lymphocyte function-associated antigen
- IAP
integrin-associated protein
- MAC
membrane attack complex
References
- 1.Mariscalco M M, Smith C W. Fetal and Neonatal Physiology. Philadelphia: Saunders; 1997. , in press. [Google Scholar]
- 2.Springer T. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 3.Bessis M. Nouv Rev Fr Hematol. 1973;13:285–290. [PubMed] [Google Scholar]
- 4.Malawista S E, de Boisfleury Chevance A. J Leukocyte Biol. 1997;61:58–62. doi: 10.1002/jlb.61.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Malawista S E, de Boisfleury Chevance A. J Leukocyte Biol. 1991;50:313–315. doi: 10.1002/jlb.50.3.313. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Schonbein G W, Shih Y Y, Chien S. Blood. 1980;56:866–875. [PubMed] [Google Scholar]
- 7.Brown E J, Lindberg F P. Ann Med. 1996;28:201–208. doi: 10.3109/07853899609033121. [DOI] [PubMed] [Google Scholar]
- 8.Kubes P, Niu X-F, Smith C W, Kehrli M E, Jr, Reinhardt P, Woodman R. FASEB. 1995;9:1103–1111. [PubMed] [Google Scholar]
- 9.Bainton D F, Miller L J, Kishimoto T K, Springer T A. J Exp Med. 1987;166:1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller L J, Bainton D F, Borregaard N, Springer T A. J Clin Invest. 1987;80:535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson D, Miller L, Schmalstieg F, Rothlein R, Springer T. J Immunol. 1986;137:15–27. [PubMed] [Google Scholar]
- 12.Schmalstieg F C, Rudloff H E, Hillman G R, Anderson D C. J Leukocyte Biol. 1986;40:677–691. doi: 10.1002/jlb.40.6.677. [DOI] [PubMed] [Google Scholar]
- 13.Brown A F. J Cell Sci. 1982;58:455–467. doi: 10.1242/jcs.58.1.455. [DOI] [PubMed] [Google Scholar]
- 14.Lackie J M, Wilkinson P C. In: White Cell Mechanics. Meiselman H J, Lichtman M A, LaCelle P L, editors; Meiselman H J, Lichtman M A, LaCelle P L, editors. New York: Liss; 1984. pp. 237–254. [Google Scholar]
- 15.Mandeville J T H, Lawson M A, Maxfield F R. J Leukocyte Biol. 1997;61:188–200. doi: 10.1002/jlb.61.2.188. [DOI] [PubMed] [Google Scholar]
- 16.Davis G. Exp Cell Res. 1992;200:242–252. doi: 10.1016/0014-4827(92)90170-d. [DOI] [PubMed] [Google Scholar]
- 17.Anderson D, Springer T. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 18.Doerschuk C, Winn R, Coxson H, Harlan J. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- 19.Bullard D C, Qin L, Lorenzo I, Quinlan W M, Doyle N A, Bosse R, Vestweber D, Doerschuk C M, Beaudet A L. J Clin Invest. 1995;95:1782–1788. doi: 10.1172/JCI117856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lien D C, Henson P M, Capen R L, Henson J E, Hanson W L, Wagner W W, Jr, Worthen G S. Lab Invest. 1991;65:145–159. [PubMed] [Google Scholar]
- 21.Guntheroth W G, Luchtel D L, Kawabori I. J Appl Physiol. 1982;53:510–515. doi: 10.1152/jappl.1982.53.2.510. [DOI] [PubMed] [Google Scholar]