Abstract
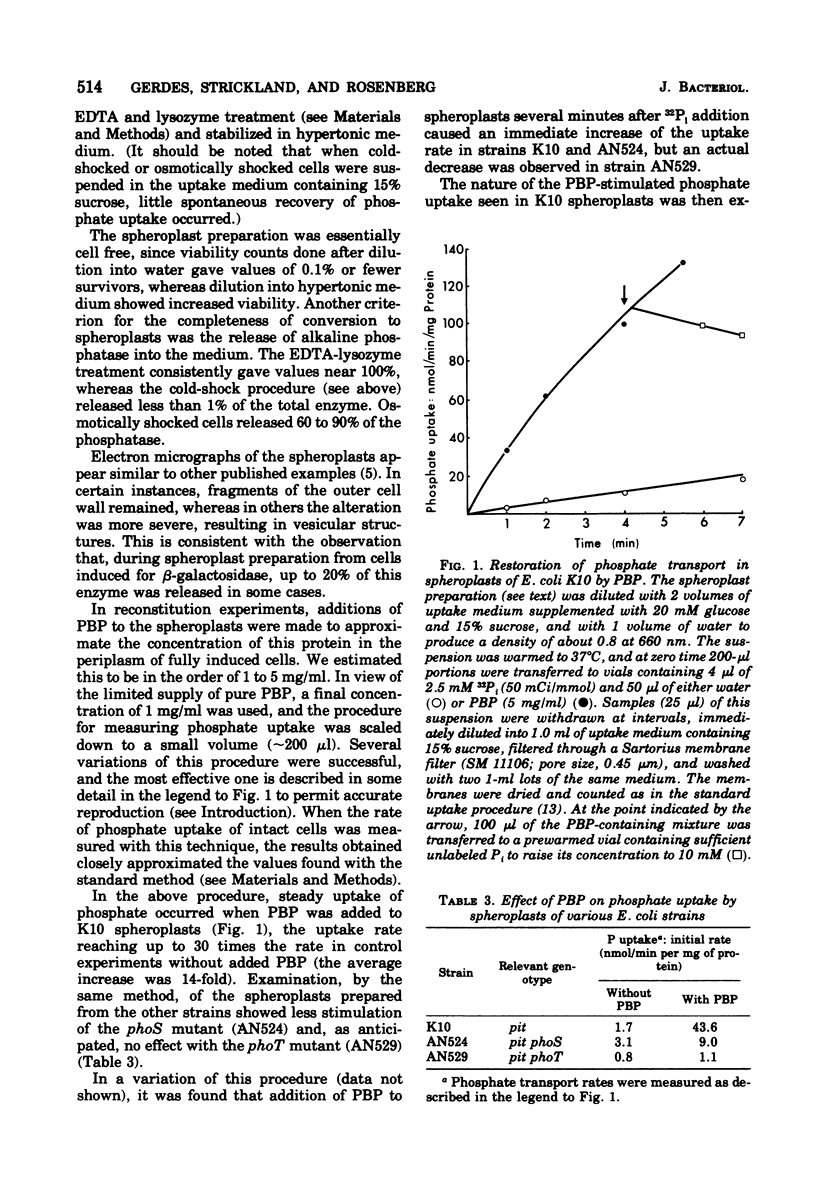
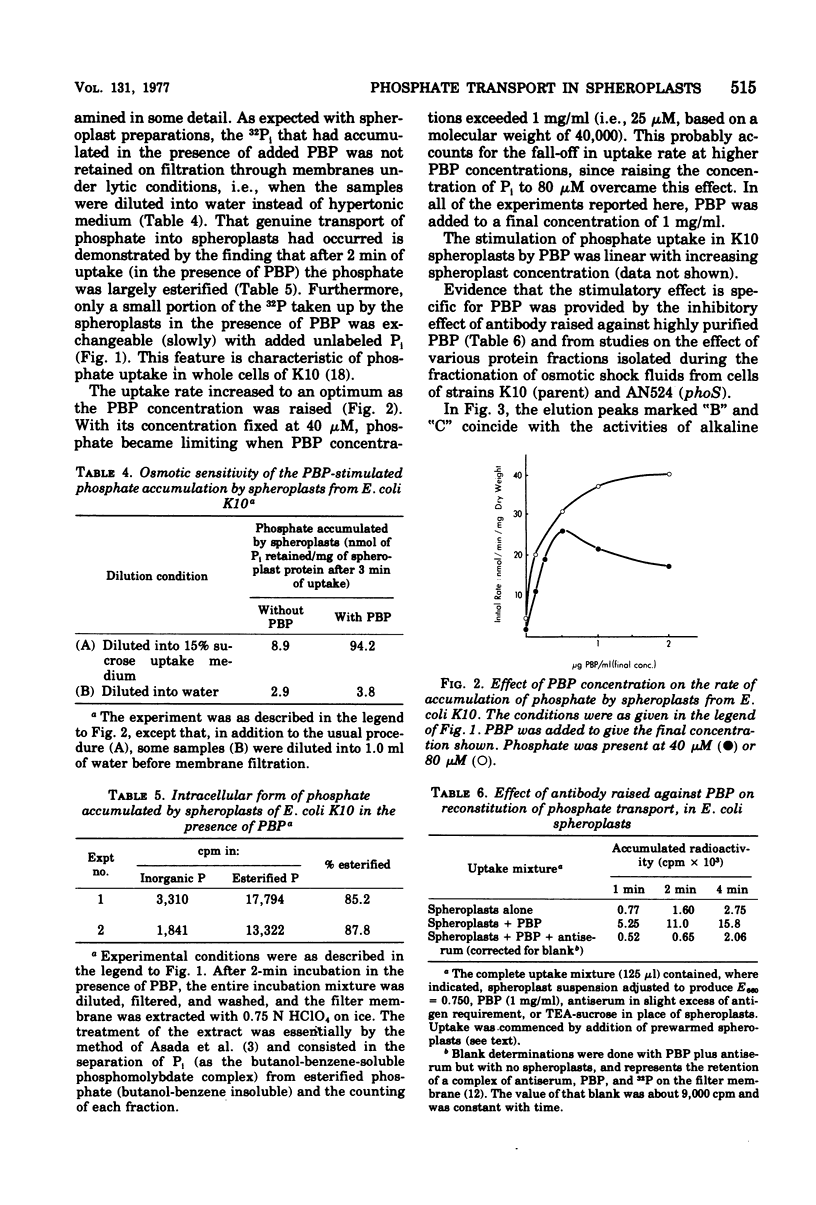
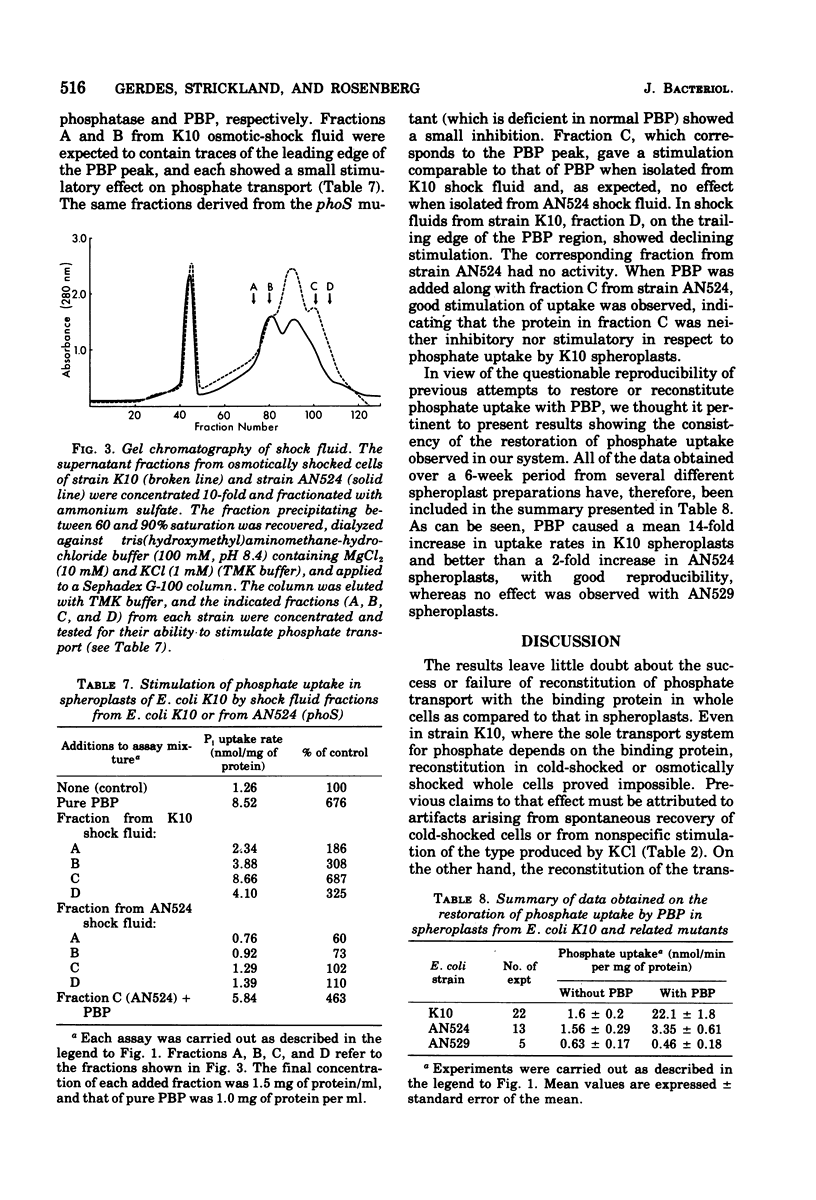
Reconstitution of phosphate transport in Escherichia coli was demonstrated. Conversion of E. coli K10 cells to spheroplasts decreased phosphate transport to about 2%. Addition of purified phosphate-binding protein at physiological levels to these spheroplasts caused a mean 14-fold increase in phosphate transport rate. Crude shock fluid fractions were also stimulatory but not if the shock fluid was obtained from mutants lacking phosphate-binding protein. The effect of the binding protein was abolished by its specific antibody. The phosphate was shown to have entered the cell, where it became esterified. Reconstitution was not possible with cold-shocked or osmotically shocked cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y., Kobayashi H., Amanuma H., Yamaguchi A. Transport of sugars and amino acids in bacteria. VII. Characterization of the reaction of restoration of active transport mediated by binding protein. J Biochem. 1973 Dec;74(6):1249–1261. doi: 10.1093/oxfordjournals.jbchem.a130353. [DOI] [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. 3. Studies on the restoration of active transport. J Biol Chem. 1968 Jun 10;243(11):3128–3135. [PubMed] [Google Scholar]
- Asada K., Takahashi M., Urano M. Phosphorylation assay in liquid scintillation counter using Cerenkov radiation of 32 P: application to photophosphorylation. Anal Biochem. 1972 Jul;48(1):311–315. doi: 10.1016/0003-2697(72)90195-9. [DOI] [PubMed] [Google Scholar]
- Barash H., Halpern Y. S. Glutamate-binding protein and its relation to glutamate transport in Escherichia coli K-12. Biochem Biophys Res Commun. 1971 Nov 5;45(3):681–688. doi: 10.1016/0006-291x(71)90470-0. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Davis B. D. The Isolation of Biochemically Deficient Mutants of Bacteria by Means of Penicillin. Proc Natl Acad Sci U S A. 1949 Jan;35(1):1–10. doi: 10.1073/pnas.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., OTSUJI N. ISOLATION OF A PROTEIN SPECIFIED BY A REGULATOR GENE. J Mol Biol. 1964 Jun;8:841–852. doi: 10.1016/s0022-2836(64)80165-0. [DOI] [PubMed] [Google Scholar]
- Gerdes R. G., Rosenberg H. The relationship between the phosphate-binding protein and a regulator gene product from Escherichia coli. Biochim Biophys Acta. 1974 May 10;351(1):77–86. doi: 10.1016/0005-2795(74)90066-x. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Quantitative assay of the binding of small molecules to protein: comparison of dialysis and membrane filter assays. Anal Biochem. 1972 Nov;50(1):73–83. doi: 10.1016/0003-2697(72)90487-3. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J. EFFECT OF INTEGRATED SEX FACTOR ON TRANSDUCTION OF CHROMOSOMAL GENES IN ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:680–686. doi: 10.1128/jb.89.3.680-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae A. S., Strickland K. P., Medveczky N., Rosenberg H. Studies of phosphate transport in Escherichia coli. I. Reexamination of the effect of osmotic and cold shock on phosphate uptake and some attempts to restore uptake with phosphate binding protein. Biochim Biophys Acta. 1976 May 21;433(3):555–563. doi: 10.1016/0005-2736(76)90281-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977 Aug;131(2):505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Bell R. M., Cronan J. E., Jr A mutant of Escherichia coli auxotrophic for organic phosphates: evidence for two defects in inorganic phosphate transport. Mol Gen Genet. 1975 Dec 30;143(1):71–77. doi: 10.1007/BF00269422. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Stimulation of arginine transport in osmotically shocked Escherichia coli W cells by purified arginine-binding protein fractions. J Biol Chem. 1969 May 25;244(10):2743–2749. [PubMed] [Google Scholar]



