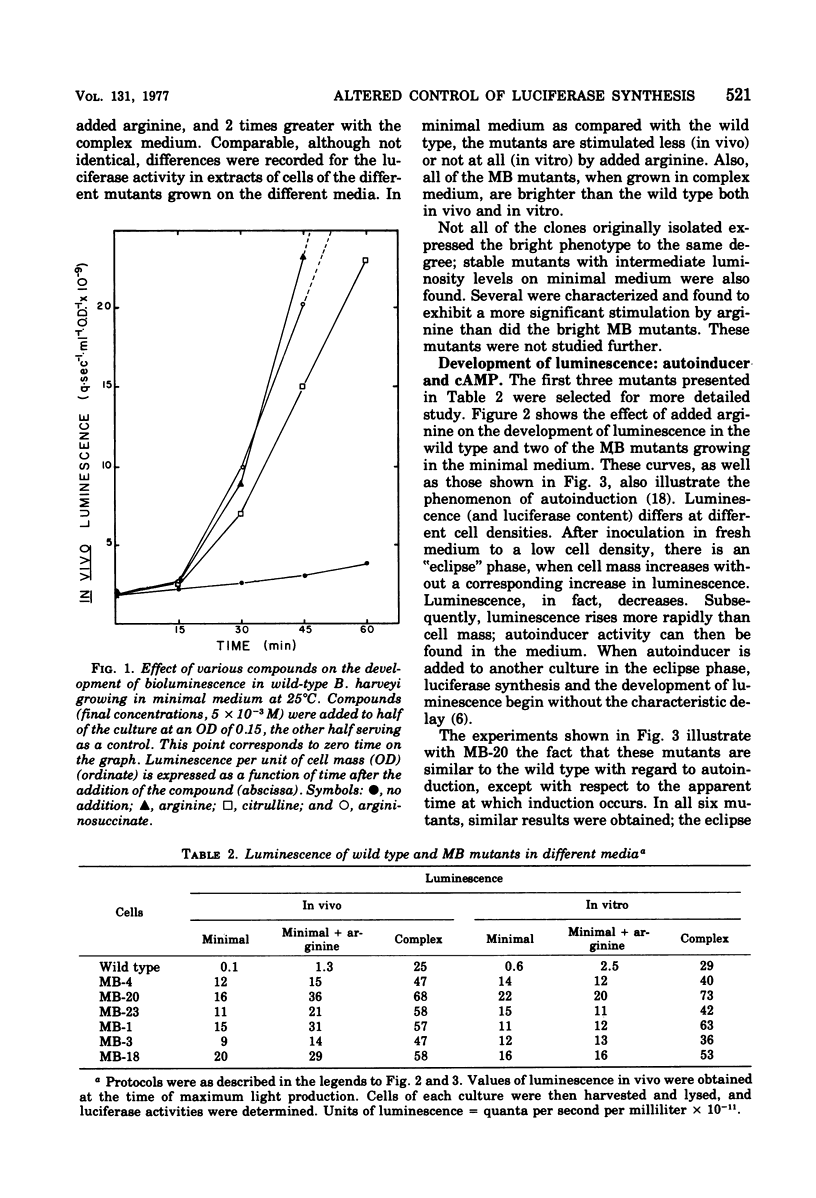
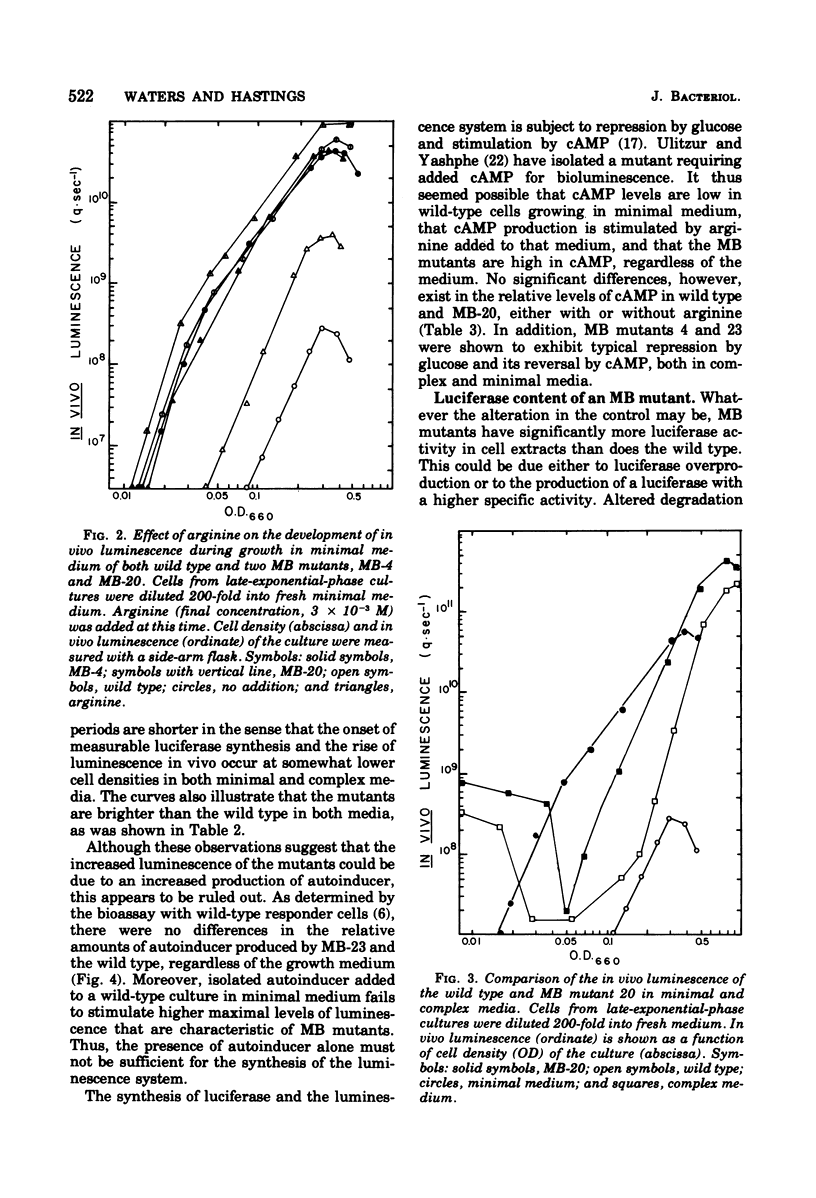
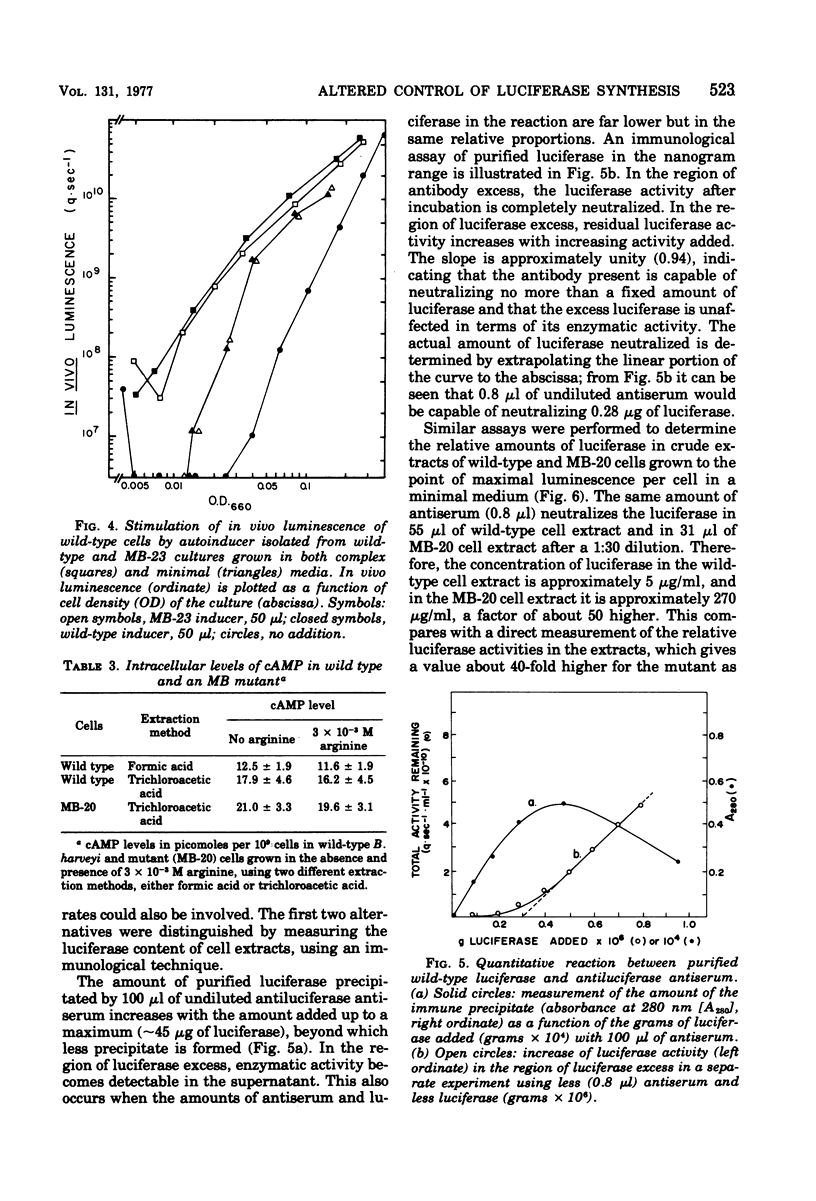
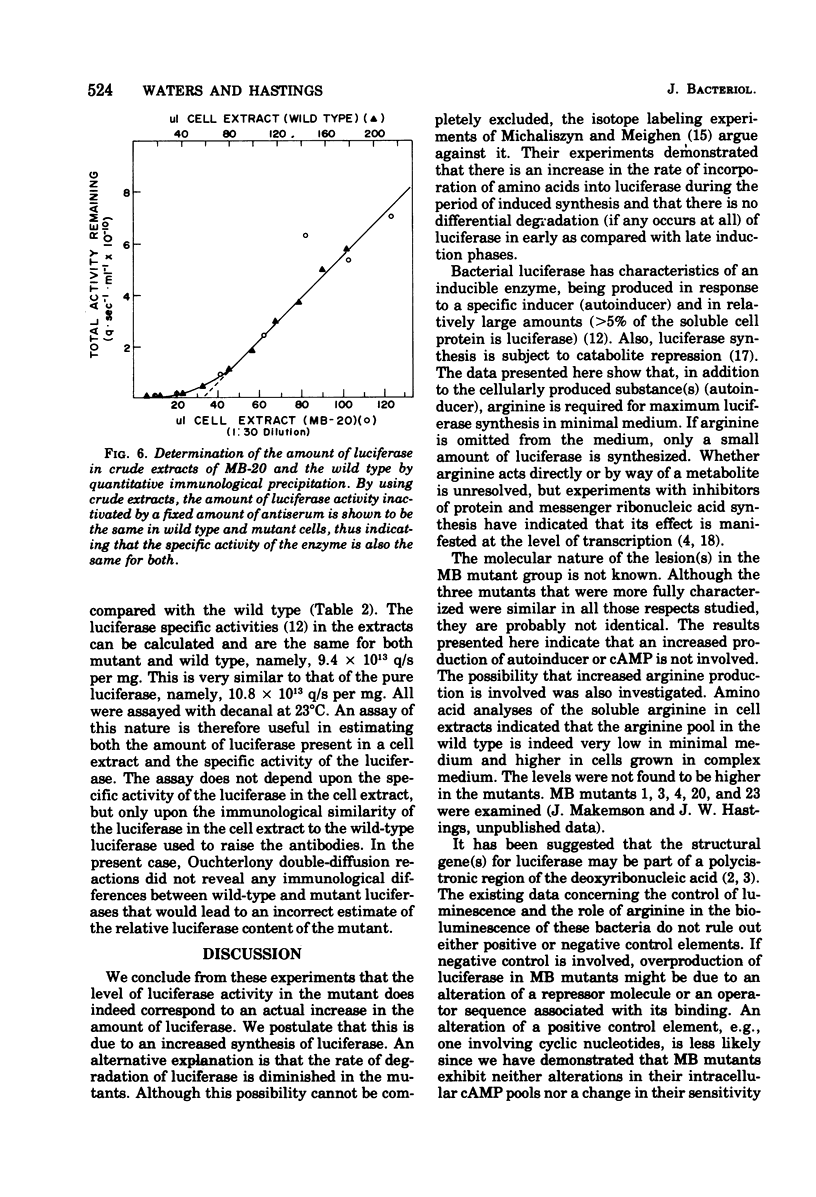
Abstract
Arginine is known to increase the luminescence in vivo and in vitro of the marine bacterium Beneckea harveyi growing in minimal medium. Mutants in which this arginine effect is either diminished, or absent were isolated as bright clones on a minimal medium after N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis. On a minimal medium both with and without added arginine and also on complex medium, these "minimal bright" mutants produce higher levels of luminescence than the wild type both in vivo and in vitro. This is attributed to the production of an increased amount of luciferase, which itself is wild type in terms of its specific activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. O., Nicoli M. Z., Becvar J. E., Hastings J. W. Bacterial luciferase. Binding of oxidized flavin mononucleotide. J Biol Chem. 1975 Apr 25;250(8):2763–2768. [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Bacterial bioluminescence in vivo: control and synthesis of aldehyde factor in temperature-conditional luminescence mutants. J Bacteriol. 1974 Jun;118(3):1059–1066. doi: 10.1128/jb.118.3.1059-1066.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Mutationally altered bacterial luciferase. Implications for subunit functions. Biochemistry. 1972 Aug 29;11(18):3359–3370. doi: 10.1021/bi00768a008. [DOI] [PubMed] [Google Scholar]
- Coffey J. J. Inducible synthesis of bacterial luciferase: specificity and kinetics of induction. J Bacteriol. 1967 Nov;94(5):1638–1647. doi: 10.1128/jb.94.5.1638-1647.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus-Miguel A., Meighen E. A., Nicoli M. Z., Nealson K. H., Hastings J. W. Purification and properties of bacterial luciferases. J Biol Chem. 1972 Jan 25;247(2):398–404. [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem. 1963 Jul;238:2537–2554. [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- Hastings J. W. Bioluminescence: from chemical bonds to photons. Ciba Found Symp. 1975;(31):125–146. doi: 10.1002/9780470720134.ch8. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Hanson F. E. Aspects of light production by Photobacterium fischeri. J Bacteriol. 1968 Mar;95(3):975–979. doi: 10.1128/jb.95.3.975-979.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaliszyn G. A., Meighen E. A. Induced polypeptide synthesis during the development of bacterial bioluminescence. J Biol Chem. 1976 May 10;251(9):2541–2549. [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Eberhard A., Hastings J. W. Catabolite repression of bacterial bioluminescence: functional implications. Proc Natl Acad Sci U S A. 1972 May;69(5):1073–1076. doi: 10.1073/pnas.69.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2324–2328. doi: 10.1073/pnas.71.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Kohama Y. Reactions involved in bioluminescence systems of limpet (Latia neritoides) and luminous bacteria. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2086–2089. doi: 10.1073/pnas.69.8.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Yashphe J. An adenosine 3',5'-monophosphate-requiring mutant of the luminous bacteria Beneckea harveyi. Biochim Biophys Acta. 1975 Oct 9;404(2):321–328. doi: 10.1016/0304-4165(75)90339-6. [DOI] [PubMed] [Google Scholar]


