Abstract
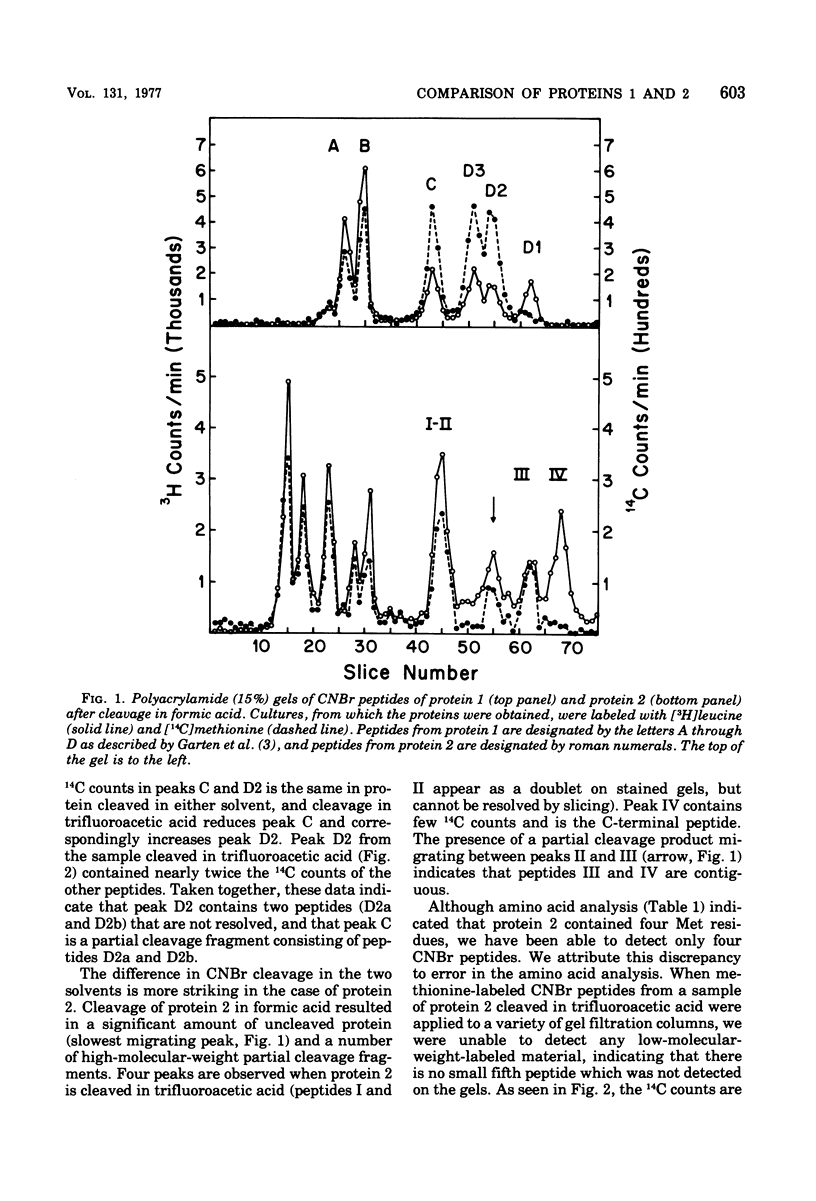
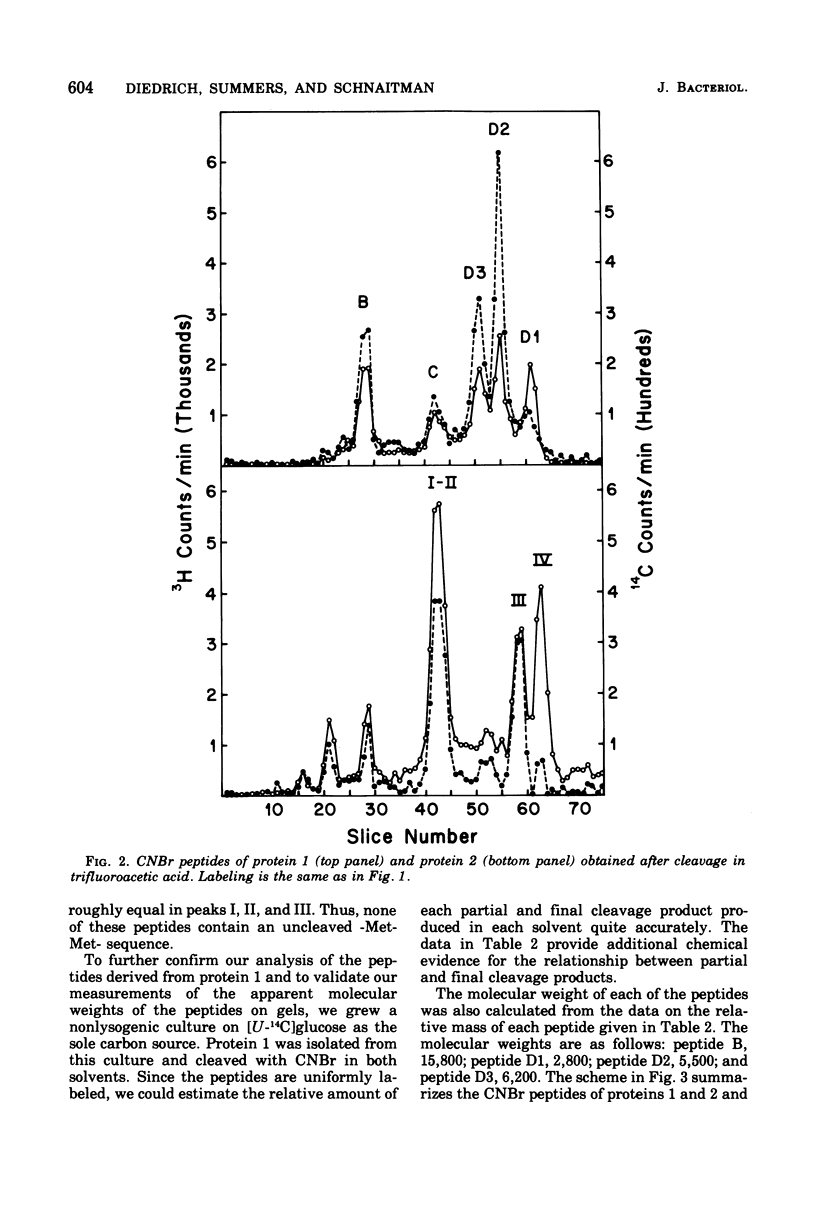
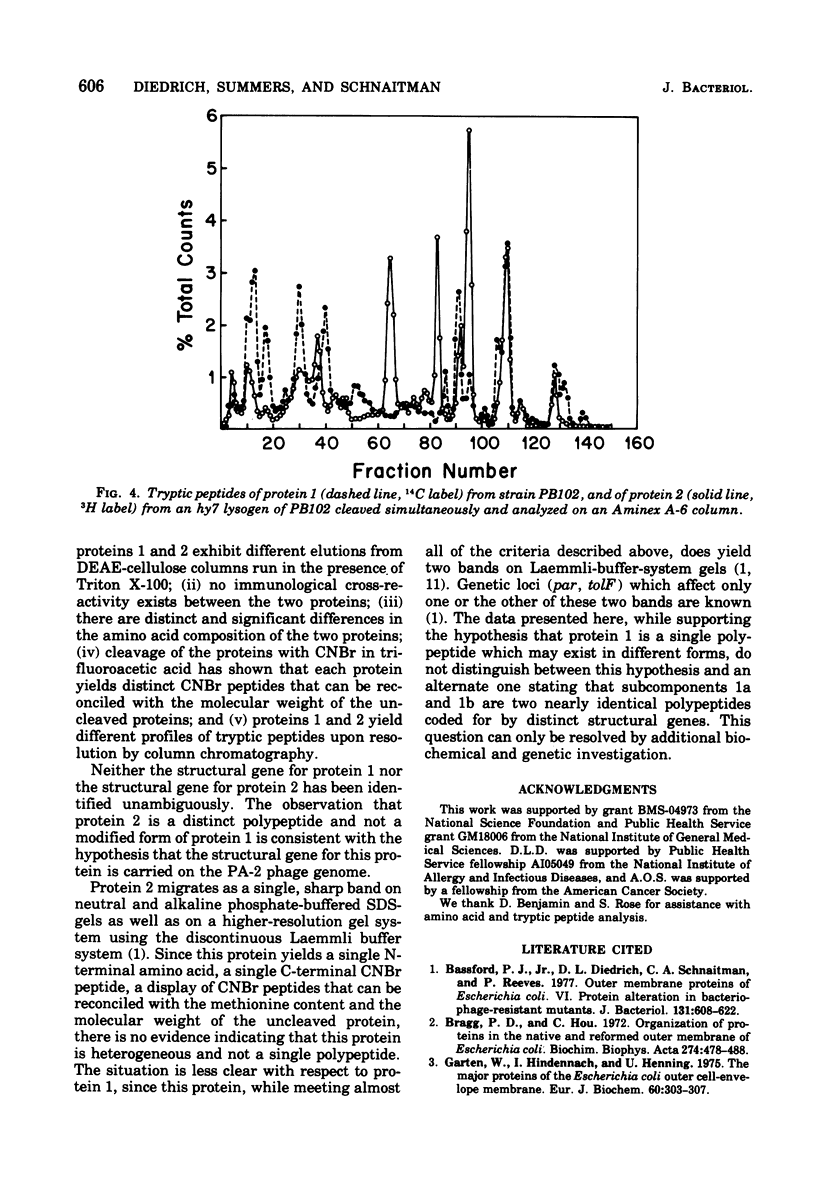
Protein 1 from the outer membrane of Escherichia coli K-12 and protein 2 from a phage PA-2 lysogen of the same strain were isolated by differential sodium dodecyl sulfate extraction and purified by ion-exchange and gel filtration chromatography. Rabbit antisera were prepared against these proteins and showed no cross-reaction between proteins 1 and 2. The proteins have the same N-terminal amino acid but show small yet significant differences in amino acid composition. The proteins were cleaved with cyanogenbromide in solvents containing both formic acid and trifluoroacetic acid. By comparing the cleavage in these solvents, it was established that protein 1 yielded 5 cyanogen bromide peptides, and the sum of the molecular weights of these was equivalent to the molecular weight of the uncleaved protein. Protein 2 yielded 4 cyanogen bromide peptides, none of which was identical to those of protein 1, and the sum of these peptides was also equivalent to the apparent molecular weight of the uncleaved protein. Significant differences were also observed when tryptic peptides from the two proteins were compared. These results indicate that protein 1 and the phage-directed protein 2 are distinct, different, and apparently homogeneous proteins.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Cyanogen bromide fragments of protein I, composition and order. Eur J Biochem. 1975 Dec 1;60(1):303–307. doi: 10.1111/j.1432-1033.1975.tb21004.x. [DOI] [PubMed] [Google Scholar]
- Haller I., Henning U. Cell envelope and shape of Escherichia coli K12. Crosslinking with dimethyl imidoesters of the whole cell wall. Proc Natl Acad Sci U S A. 1974 May;71(5):2018–2021. doi: 10.1073/pnas.71.5.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas W. K. Mapping of genes involved in the synthesis of spermidine in Escherichia coli. Mol Gen Genet. 1972;119(1):1–9. doi: 10.1007/BF00270439. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schmitges C. J., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Heterogeneity of protein I. Eur J Biochem. 1976 Mar 16;63(1):47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. II. Heterogeneity of major outer membrane polypeptides. Arch Biochem Biophys. 1973 Aug;157(2):553–560. doi: 10.1016/0003-9861(73)90674-7. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. B., Shelton J. R. An examination of conditions for the cleavage of polypeptide chains with cyanogen bromide: application to catalase. Arch Biochem Biophys. 1969 Mar;130(1):551–556. doi: 10.1016/0003-9861(69)90069-1. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



